Application Note 1014
AN70125_E 08/12S
Australia
+61 3 9757 4486
Austria
+43 1 616 51 25
Benelux
+31 20 683 9768
+32 3 353 42 94
Brazil
+55 11 3731 5140
China
+852 2428 3282
Denmark
+45 36 36 90 90
France
+33 1 39 30 01 10
Germany
+49 6126 991 0
India
+91 22 2764 2735
Ireland
+353 1 644 0064
Italy
+39 02 51 62 1267
Japan
+81 6 6885 1213
Korea
+82 2 3420 8600
Singapore
+65 6289 1190
Sweden
+46 8 473 3380
Switzerland
+41 62 205 9966
Taiwan
+886 2 8751 6655
UK
+44 1276 691722
USA and Canada
+847 295 7500
©2012 Thermo Fisher Scientific Inc. All rights reserved. ISO is a trademark of the International Standards Organization.
All other trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. This information is presented as
an example of the capabilities of Thermo Fisher Scientific Inc. products. It is not intended to encourage use of these products
in any manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to
change. Not all products are available in all countries. Please consult your local sales representative for details.
Thermo Scientific Dionex products are
designed, developed, and manufactured
under an ISO 9001 Quality System.
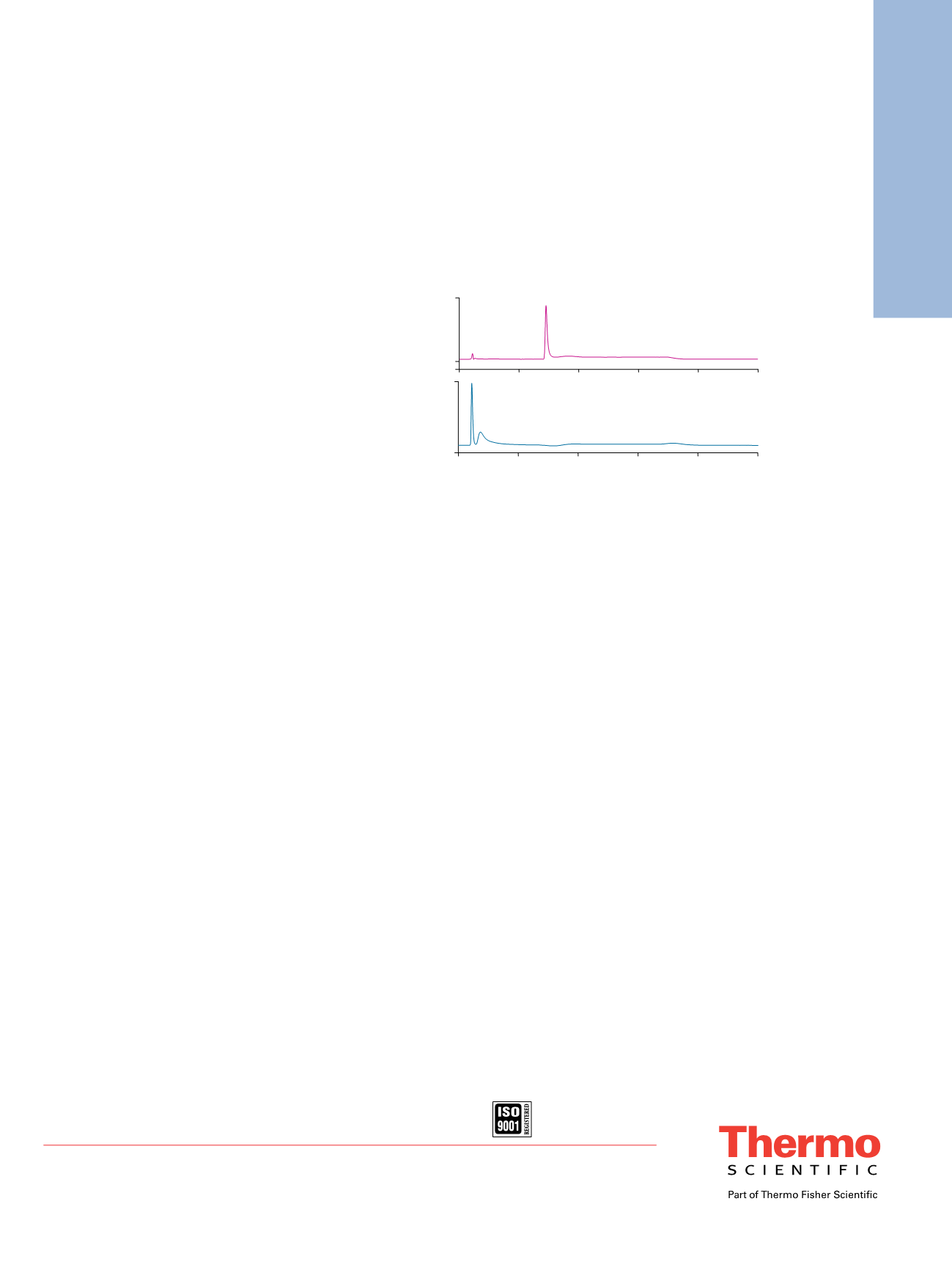
Endo H Digestion Eliminates the Con A Binding
Ability of HRP
Endo H has been reported to cleave within the chitobiose
core of high-mannose type and some hybrid type oligosac-
charides from
N
-linked glycoproteins. Figure 6 shows
that HRP cannot be retained by the ProSwift ConA-1S
Affinity column after Endo H treatment. This observation
confirms that main glycosylation types of HRP are high
mannose and/or hybrid. Peak 3 in Figure 6 is postulated
to be either the released oligosaccharides—because it is
not found in the Endo H control (no HRP added)—or
more likely, the fraction of glycosylated HRP that is
not susceptible to Endo H (i.e., all Endo H-susceptible
structures have been removed and only the nonsusceptible
structures remain on the HRP). It is more likely a
non-Endo H-susceptible fraction because oligosaccharides
have little or no absorbance at 210 nm.
Conclusion
This work shows that the HPLC-compatible ProSwift
ConA-1S Affinity column can capture glycoproteins and
glycopeptides efficiently. The UltiMate 3000 ×2 Dual
Biocompatible Analytical LC system can automate the
entire off-line 2D process from ProSwift ConA-1S Affinity
column sample enrichment and fraction collection to
automatic reanalysis of collected sample by peptide
mapping. Monitoring oxonium ions (e.g.,
m/z
204, 366,
and 163) in a peptide mixture with a single quadrupole
mass spectrometer is a selective, sensitive, and reliable
method that also confirms the identity of glycopeptides
captured by the ProSwift ConA-1S Affinity column.
References
1.Dionex (now part of Thermo Scientific) Application
Update 183: Separation of Peptides from Enzymatic
Digestion on Different Acclaim Columns: A
Comparative Study. Sunnyvale, CA, 2011. [Online]
-
Peptides-EnzyDigest-AcclaimCompar-02Nov2011-
LPN2973.pdf (accessed June 12, 2012).
2.Harvey, D.J.; Wing, D.R.; Küster, B.; Wilson, R.B.H.
Composition of N-Linked Carbohydrates from
Ovalbumin and Co-purified Glycoproteins.
J. Am. Soc.
Mass Spectrom.
2000
,
11
, 564–571.
3.Wuhrer, M.; Hokke, C.H.; Deelder, A.M. Glycopeptide
Analysis by Matrix-Assisted Laser Desorption/
Ionization Tandem Time-of-Flight Mass Spectrometry
Reveals Novel Features of Horseradish Peroxidase
Glycosylation: A Correlation Study.
Rapid Commun.
Mass Spectrom.
2004
,
18
, 1741–1748.
4. Bean, M.F.; Annan, R.S.; Hemling, M.E.; Mentzer, M.;
Huddleston, M.J.; Carr, S.A. LC-MS Methods for
Selective Detection of Posttranslational Modifications in
Proteins: Glycosylation, Phosphorylation, Sulfation,
and Acylation. In
Techniques in Protein Chemistry
;
J. Crabb, ed.; Academic Press: San Diego, 1995; pp
107–116.
5.Wuhrer, M.; Balog, C.R.A.; Koeleman, C.A.M; Deelder,
A.M; Hokke, C.H. New Features of Site-Specific
Horseradish Peroxidase (HRP) Glycosylation
Uncovered by nano-LC-MS with Repeated Ion-
Isolation/Fragmentation Cycles.
Biochim. Biophys. Acta
2005
,
1723
, (1-3) 229–239.
Figure 6. Glycosylated HRP enrichment on the ProSwift ConA-1S Affinity column
(A) before and (B) after Endo H treatment.
A
1
2
B
3
2
-50
400
0
5
10
15
20
25
mAU
Minutes
300
-50
mAU
Column:
ProSwift ConA-1S Affinity (5 × 50 mm)
Mobile Phase:
A: 50 mM sodium acetate, 200 mM sodium chloride, 1 mM calcium
chloride, pH 5.3
B: 100 mM
α
-methyl mannoside in mobile phase A
Gradient:
0–5.0 min, 0% B; 5.0–5.5 min,
0–100% B; 5.5–15 min, 100% B
Flow Rate:
0.5 mL/min
Inj. Volume:
20 µL
Temperature:
30 °C
Detection:
UV at 214 nm
Sample Preparation: 1 mg/mL HRP in water/mobile phase A
Samples:
A
. Before Endo H treatment
B
. After Endo H treatment
Peaks:
1. HRP
2., 3. Deglycosylated HRP


