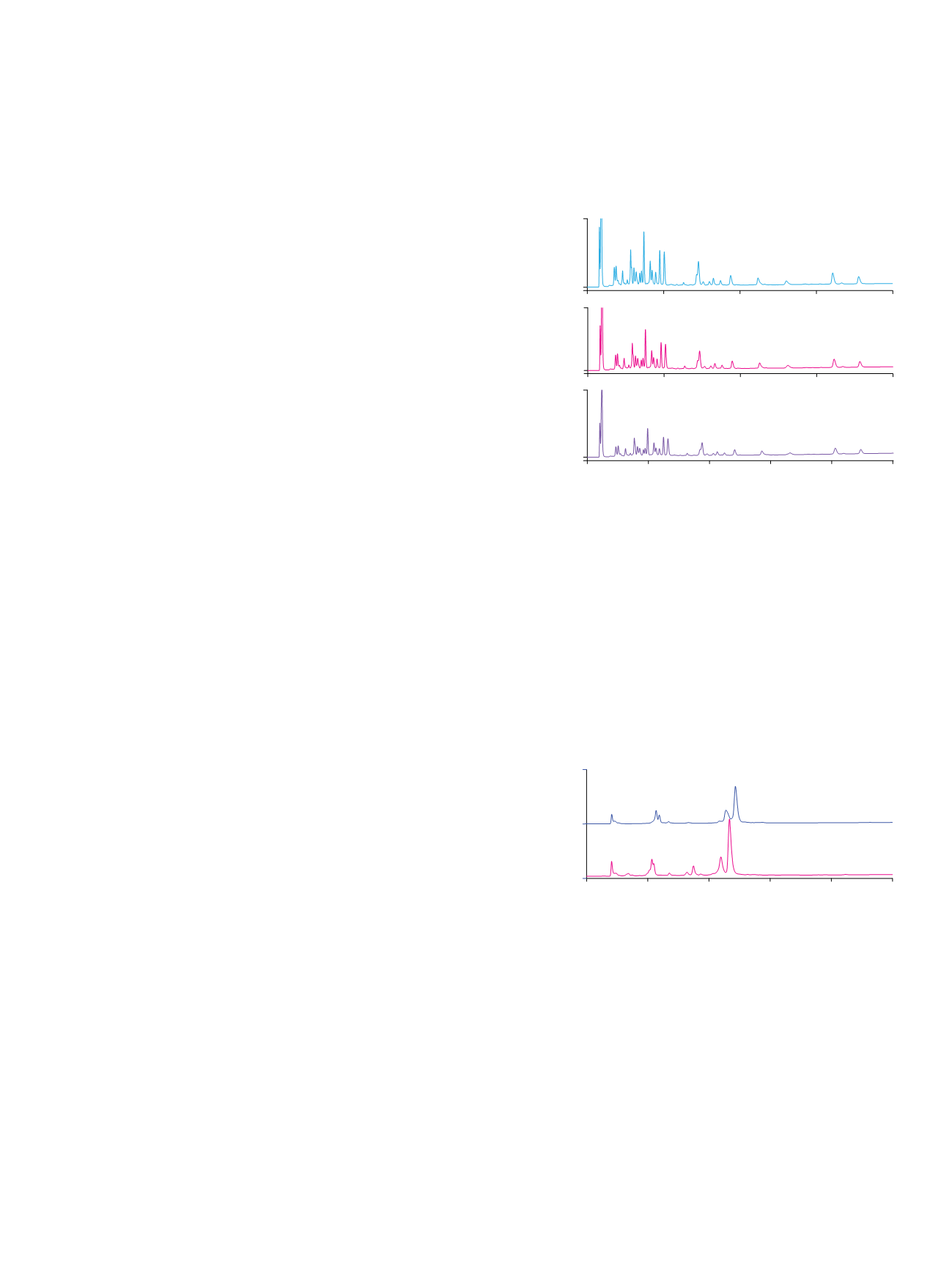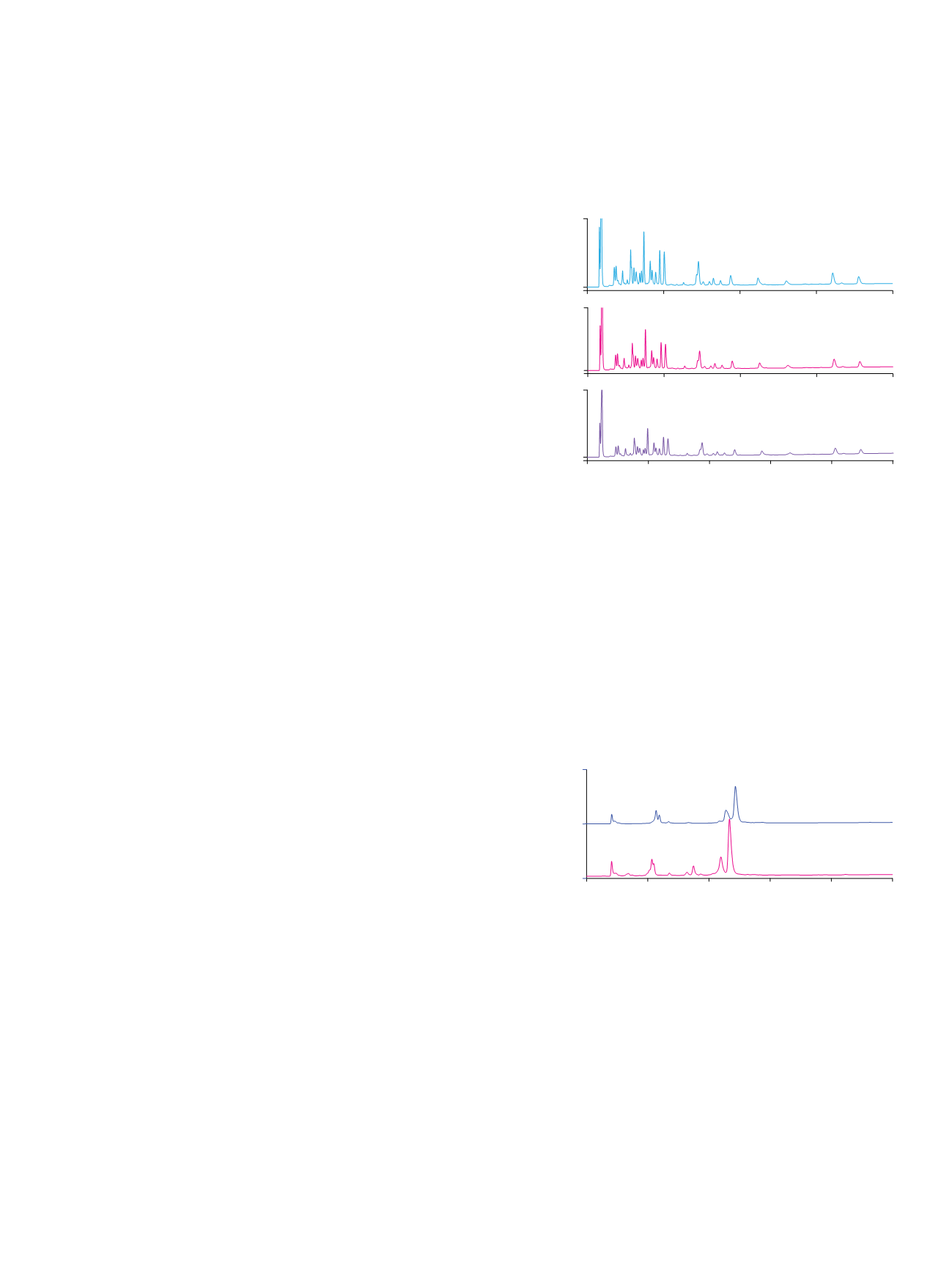
2
Preparation of Solutions
Buffers for Tryptic Digestion
For detailed methods of preparing buffers for tryptic
digestion, refer to Dionex (now part of Thermo Scientific)
Application Update (AU) 183.
3
Mobile Phases with Triethylamine Phosphate
(TEAP)
Prepare a 20 mM triethylamine (TEA) solution, then
adjust the pH of the solution to 2.0 or 3.9 using
phosphoric acid. Add acetonitrile and sodium perchlorate
to make mobile phase A or mobile phase B for the
ion-exchange separation. For example, mix the pH 2
TEAP solution 1:1 with acetonitrile to prepare mobile
phase A shown in Figure 1. To prepare mobile phase B
shown in Figure 1, prepare 1 L of mobile phase A and
dissolve 14.05 g of sodium perchlorate in that solution.
Sample Preparation
Reduce, alkylate, and dialyze myoglobin extensively
against 50 mM sodium bicarbonate. Then digest the
resulting reduced and alkylated myoglobin with trypsin
overnight. For detailed procedures, refer to AU 183.
Results and Discussion
Use of a TEAP/sodium perchlorate mobile phase with
manipulation of the pH and organic solvent modifier
concentration allowed high-resolution separation of an
equine heart myoglobin tryptic digest (Figure 1). This
separation was easily accelerated without compromising
resolution by simply increasing the flow rate. This method
can also be applied to a synthetic peptide. Figure 2 shows
similar high resolution and fast separation of a synthetic
peptide and its byproducts. In contrast to the myoglobin
tryptic digest separation, better separation was observed
at pH 3.9 than at 2.0. This may be due to the basic nature
of the synthetic peptide.
Separation on the cation-exchange column is primarily
determined by analyte charge, but hydrophilic and
hydrophobic interactions also play a role. Organic solvent
modifiers such as acetonitrile improve separation by
changing solubility, hydrophilic interaction, and possibly
peptide conformation. Sodium perchlorate was used to
elute peptides due to its better solubility in acetonitrile
and its stronger elution power compared to the commonly
used sodium chloride.
Figure 1. Chromatograms of a myoglobin tryptic digest with different flow rates.
Figure 2. Chromatograms of a synthetic peptide and its byproducts at different
mobile phase pH values.
0
7
14
21
28
100
mAU
-5
100
mAU
-5
100
mAU
-5
0
5
10
15
20
25
Minutes
0
10
20
30
40
(1)
(2)
(3)
Column:
ProPac
™
SCX-10, 10 µm (4 × 250 mm, P/N 075725)
Mobile Phase: A: 20 mM TEAP, pH 2.0, 50% acetonitrile
B: 100 mM sodium perchlorate in A
Gradient:
1
. 0–40 min, 0–100% B, at 0.7 mL/min
2
. 0–28 min, 0–100% B, at 1.0 mL/min
3
. 0–20 min, 0–100% B, at 1.4 mL/min
Inj. Volume:
20 µL
Temperature:
30 °C
Detection:
UV, 214 nm
Sample:
Myoglobin (from equine heart) tryptic digest
Sample Preparation: Reduce, alkylate, dialyze, and digest with trypsin overnight
0
2
4
6
8
10
-100
100
0 mAU
Minutes
Column:
ProPac SCX-10 10 µm (4 × 250 mm)
Mobile Phase:
1
.
A: 20 mM TEAP, pH 3.9, 50% acetonitrile
B: 100 mM perchlorate in A
2
.
A: 20 mM TEAP, pH 2.0, 50% acetonitrile
B: 100 mM perchlorate in A
Gradient:
0–10 min, 0–50% B
Flow Rate:
1.4 mL/min
Inj. Volume:
20 µL
Temperature:
30 °C
Detection:
UV, 214 nm
Sample:
A synthetic peptide and its byproducts
The sequence of the peptide: (Ac-)YNIQKESTLPLVLRLRGG (–CONH
2
)
Calculated pI of the peptide: 11.4
(2)
(1)