Application Update 188
AU70140_E 08/12S
Australia
+61 3 9757 4486
Austria
+43 1 616 51 25
Benelux
+31 20 683 9768
+32 3 353 42 94
Brazil
+55 11 3731 5140
China
+852 2428 3282
Denmark
+45 36 36 90 90
France
+33 1 39 30 01 10
Germany
+49 6126 991 0
India
+91 22 2764 2735
Ireland
+353 1 644 0064
Italy
+39 02 51 62 1267
Japan
+81 6 6885 1213
Korea
+82 2 3420 8600
Singapore
+65 6289 1190
Sweden
+46 8 473 3380
Switzerland
+41 62 205 9966
Taiwan
+886 2 8751 6655
UK
+44 1276 691722
USA and Canada
+847 295 7500
©2012 Thermo Fisher Scientific Inc. All rights reserved. ISO is a trademark of the International Standards Organization.
Sigma-Aldrich is a registered trademark of Sigma-Aldrich Co. LLC. All other trademarks are the property of Thermo Fisher
Scientific Inc. and its subsidiaries. Specifications, terms and pricing are subject to change. Not all products are available in
all countries. Please consult your local sales representative for details. This information is presented as an example of the
capabilities of Thermo Fisher products. It is not intended to encourage use of these products in any manners that might infringe
the intellectual property rights of others.
Thermo Scientific Dionex products are
designed, developed, and manufactured
under an ISO 9001 Quality System.
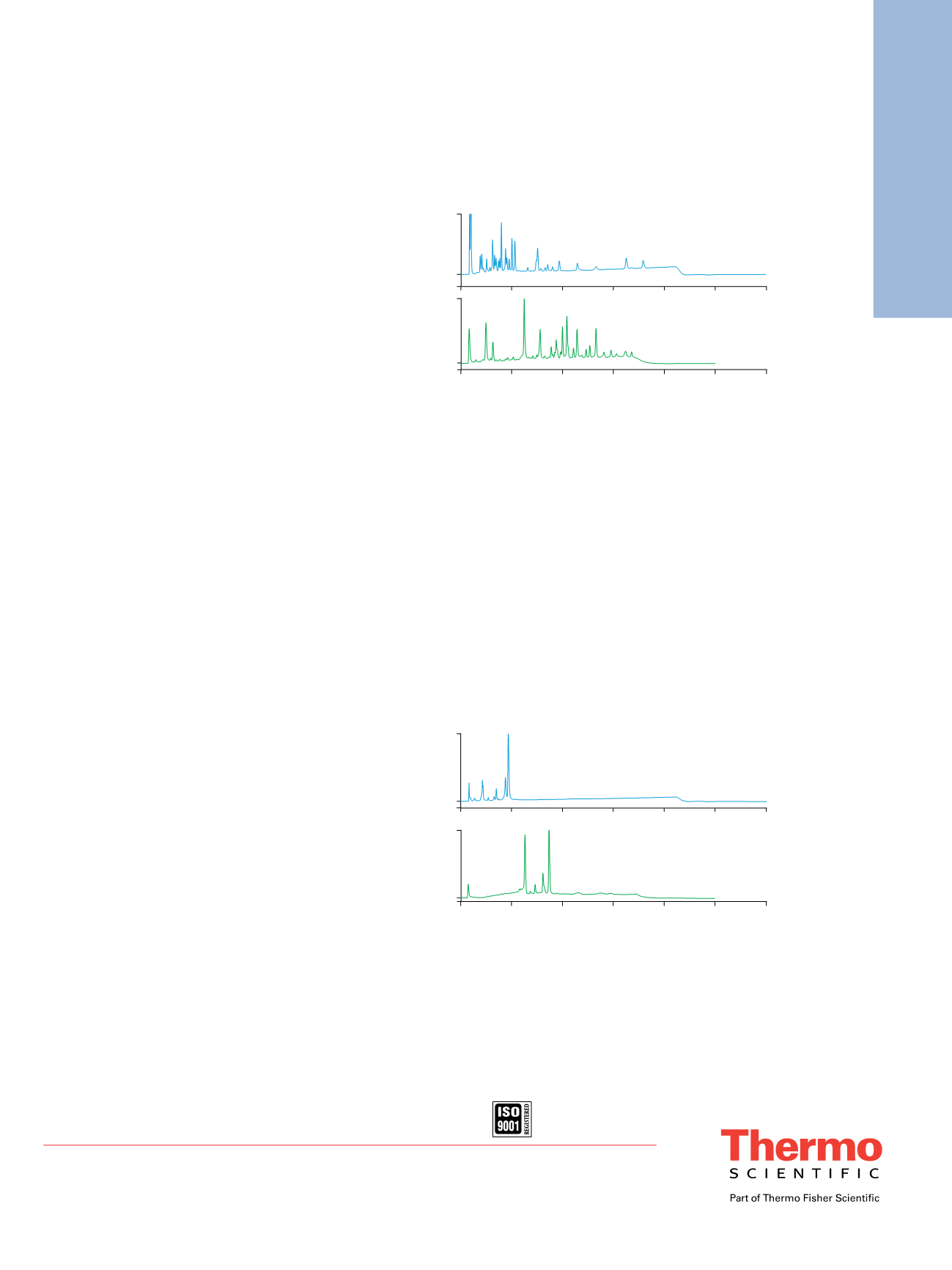
With the next-generation MabPac
™
SCX-10 (3 µm)
column, both separation efficiency and resolution can be
further improved (Figure 5). Separation time can be easily
reduced to <10 min by simply increasing the flow rate.
Additionally, the peptides have better retention and are
better resolved on the MabPac SCX-10 column compared
to the ProPac SCX-10 column.
For the synthetic peptide and byproducts separation, the
ProPac SCX-10 and MabPac SCX-10 (3 µm) columns
provide similar resolution (Figure 6). The latter column
performs better for resolving the main product (Peak 1)
and a nearby byproduct (Peak 2).
Conclusion
This work shows that the ProPac SCX-10 column
delivers high-resolution separations for peptide mapping
and provides an alternate or supplementary method
for reversed-phase peptide separation. The new
MabPac SCX-10 (3 µm) column delivers faster high-
resolution peptide mapping. Both columns can be used to
separate a synthetic peptide and its byproducts, providing
an alternative to reversed-phase separation.
References
1.Dionex (now part of Thermo Scientific) Application
Note 521: Automated 2D LC Coupled to ESI-MS/MS
for the Analysis of Complex Peptide Samples.
Sunnyvale, CA, 2002. [Online]
webdocs/5476-AN521_LPN1470.pdf (accessed June
18, 2012).
2.Dionex (now part of Thermo Scientific) Application
Note 126: Determination of Hemoglobin Variants by
Cation-Exchange Chromatography. Sunnyvale, CA,
2007. [Online]
webdocs/4466-AN126_released022707.pdf (accessed
June 18, 2012).
3.Dionex (now part of Thermo Scientific) Application
Update 183: Separation of Peptides from Enzymatic
Digestion of Different Acclaim Columns: A
Comparative Study. Sunnyvale, CA, 2011. [Online]
-
Peptides-EnzyDigest-AcclaimCompar-02Nov2011-
LPN2973.pdf (accessed June 18, 2012).
4. Dionex (now part of Thermo Scientific) Technical Note
74: High Peak Capacity Nano LC Peptide Separations
Using Long Packed Columns. Sunnyvale, CA, 2009.
[Online]
-
TN74-HPLC-Peptides-LongColumns-29Jan09-
LPN2111.pdf (accessed Aug 8, 2012).
Figure 6. Chromatograms of a synthetic peptide and its byproduct using 1) a
ProPac SCX-10 column (10 µm particle size) and 2) a MabPac SCX-10 column
(3 µm particle size)
200
mAU
-10
100
mAU
-10
0
5
10
15
20
25
30
Minutes
(1)
(2)
Columns:
1
. ProPac SCX-10, 10 µm (4 × 250 mm, P/N 075725)
2
. MabPac SCX-10, 3 µm (4 × 50 mm, P/N 077907)
Mobile Phase:
A: 20 mM TEAP, pH 3.9, 50% acetonitrile
B: 100 mM sodium perchlorate in A
Gradient:
1
. 0–20 min, 0–100% B, at 1.4 mL/min
2
. 0–15 min, 0–100% B; 15.5–25 min, 0% B, at 0.4 mL/min
Inj. Volume:
20 µL
Temperature:
30 °C
Detection:
UV, 214 nm
Sample:
A synthetic peptide and its byproducts
The sequence of the peptide:
(Ac-)YNIQKESTLPLVLRLRGG(–CONH
2
)
Calculated pI of the peptide: 11.4
Peak X is also shown in the blank, so it should not be considered a real peak.
X
2
1
2
1
Figure 5. Chromatograms of a myoglobin tryptic digest separated by 1) a
ProPac SCX-10 column (10 µm particle size) and 2) a MabPac SCX-10 column
(3 µm particle size).
100
mAU
-10
50
mAU
-10
0
5
10
15
20
25
30
Minutes
(1)
(2)
Columns:
1
. ProPac SCX-10, 10 µm (4 × 250 mm, P/N 075725)
2
. MabPac SCX-10, 3 µm (4 × 50 mm, P/N 077907)
Mobile Phase:
A: 20 mM TEAP, pH 2.0, 50% acetonitrile
B: 100 mM sodium perchlorate in A
Gradient:
1
. 0–20 min, 0–100% B, at 1.4 mL/min
2
. 0–15 min, 0–100% B; 15.5–25 min, 0% B, at 0.4 mL/min
Inj. Volume:
20 µL
Temperature:
30 °C
Detection:
UV, 214 nm
Sample:
Myoglobin (from equine heart) tryptic digest
Sample Preparation: Reduce, alkylate, dialyze, and digest with trypsin overnight


