6
Comprehensive Sequence and Post-translational Modifications Analysis of Monoclonal Antibody by Flash Digest and LC-High Resolution MS
Conclusion
A complete workflow has been developed for the fast and comprehensive sequence
and post-translational modifications analysis of monoclonal antibodies.
A 30-min digestion time demonstrated sufficient digestion efficiency of
immobilized trypsin column for IgG mAb. Good sequence coverage of native,
non-reduced IgG light and heavy chains were obtained. Further reduction and
alkylation increased sequence coverage to 100% and 97% for light and heavy
chains, respectively.
Oxidative study results show that oxidation of methionine 49, 304 and 393 in IgG
heavy chain is dose-dependent as the oxidation reaction time. However, major
glycoforms did not change as expected.
This workflow could greatly shorten the sample preparation and data analysis
time while providing great sensitivity to detect low level PTMs. Additionally, any
unintentionally incurred oxidative stresses during biopharmaceutical production
may be rapidly analyzed for impact on production.
Reference
1. Samaranayake H, Wirth T, Schenkwein D, Raty JK, Yla-Herttuala S (2009)
Challenges in monoclonal antibody-based therapies.
Ann Med
41: 322
–
331.
2. Durocher Y, Butler M (2009) Expression systems for therapeutic glycoprotein
production.
Curr Opin Biotechnol
20: 700
–
707.
n of PTMs in Oxidatively
roxide at 15, 30, 60, 90, 120
zed by LC-MS/MS. PTMs
t amino acid sites are
mmary (Table 3) from
TABLE 3. Summary of Selected Identified PTMs and Major Glycoforms of
Oxidatively Stressed IgG Heavy Chain
samples under same LC-MS
that oxidation of methionine
nt as the oxidation reaction
mple demonstrated
n was identified by isotopic
oftware. The experimental
ftware. The well matched
pectra are shown in Figure 4,
rmation of peptide with
n of methionine and
erine, aspartic acid and
e reaction time as expected.
ut not observed.
uence coverage of both IgG
1%, respectively. On the
e sequences are mapped
sequence, which greatly
resents signal intensity of
tention time of eluting
ernight digestion protocol in
and alkylated by IAA,
The sample preparation
Relative
Abundance
15min
30min
60min
90min
120min
Unquenched
M49+Oxidation
9.67%
9.51%
10.70%
10.84%
11.43%
74.30%
~M49+Double
Oxidation
0.12%
0.13%
0.15%
0.13%
0.15%
0.12%
N60+Deamidatio
n
4.34%
5.14%
4.95%
5.55%
5.25%
4.86%
M304+Oxidation
15.35%
18.50%
24.20%
30.35%
36.76%
86.69%
M393+Oxidation
13.99%
14.28%
18.53%
21.67%
25.80%
84.36%
N292+A1G0F
15.48%
16.51%
16.17%
16.86%
16.00%
16.91%
N292+A1G1F
4.04%
4.87%
5.24%
5.17%
4.99%
4.94%
N292+A2G0F
34.82%
34.35%
35.59%
33.71%
35.34%
33.60%
N292+A2G1F
36.86%
36.12%
34.54%
36.53%
34.89%
36.36%
N292+A2G2F
7.57%
7.82%
7.62%
7.11%
7.99%
7.28%
Flash Digest is a trademark of Perfinity Biosciences Inc. ACQUITY is a trademark of Waters Corporation. All other
trademarks are the property of Thermo Fisher Scientific and its subsidiaries.
This information is not intended to encourage use of these products in any manners that might infringe the
intellectual property rights of others.
PO64086-EN 0614S
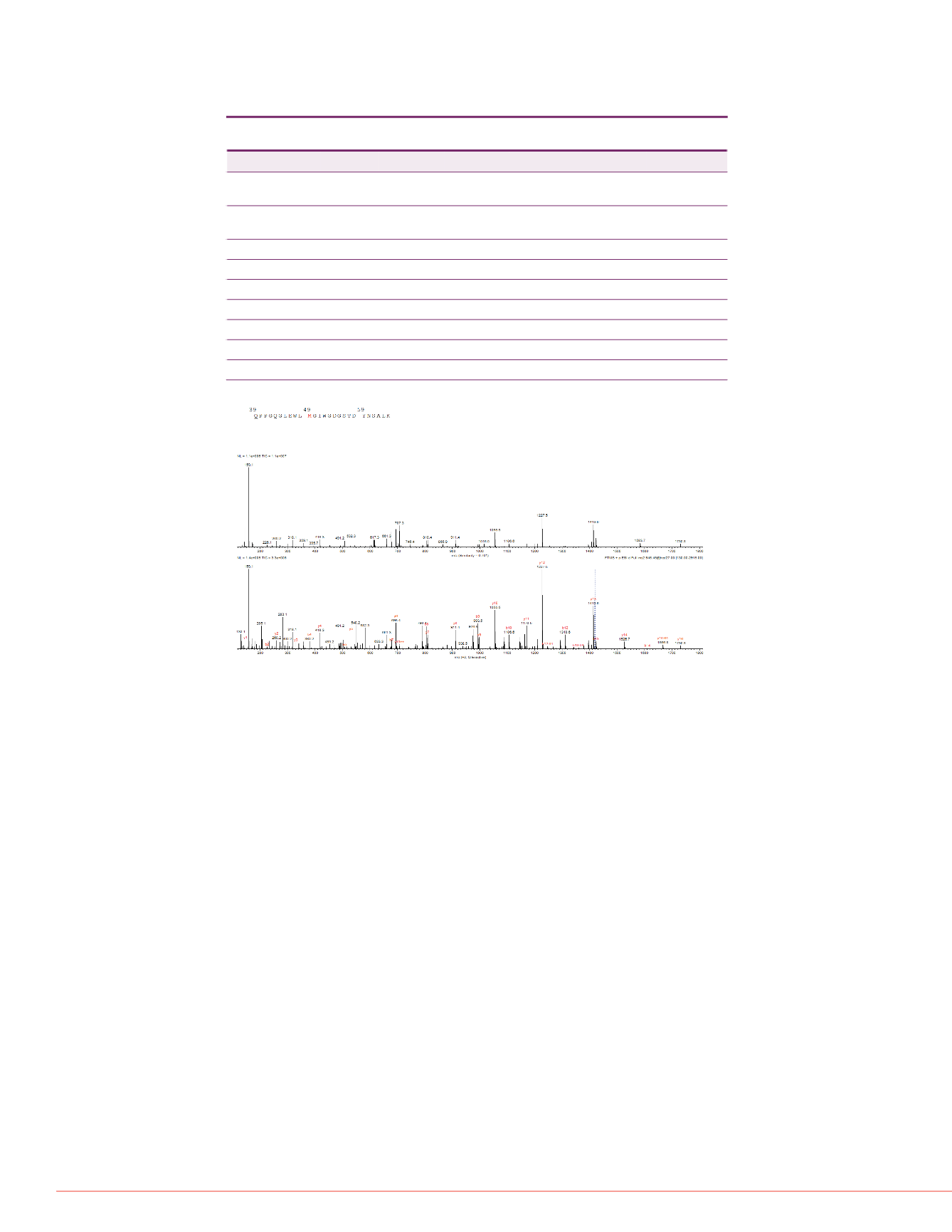
ted, Reduced and Alkylated
FIGURE 4. MS/MS Spectra of Triply Charged Peptide with Methionine Oxidation
Predicted MS/MS Spectrum
Annotated Experimental MS/MS Spectrum


