5
Figure 5. Peptide mapping of (A) HRP tryptic peptides, (B) Con A captured
fraction of HRP tryptic peptides, and (C) Con A flow-through fraction of HRP
tryptic peptides.
Selective Monitoring of Glycopeptides by
Monitoring Oxonium Ions
SIM scanning of glycan diagnostic oxonium ions and
precursor ion scanning are two frequently used methods
for selective detection of peptides with a post-translational
modification such as glycosylation and phosphorylation.
4
Without the ability to do precursor ion scanning,
scanning oxonium ions is the choice when using a single
quadrupole mass spectrometer. It is reported that
m/z
163,
204, 292, and 366 are marker ions for glycosylation.
4
Production of marker ions is controlled by the extent
of collisional excitation, which depends on the voltage
applied to the sampling cone. Maximum yield of marker
for glycosylation is reportedly generated at a cone voltage
of 140 V, which was applied in this study.
As Figure 4 shows, peak number, shape, and retention
time in the mass spectroscopy traces for
m/z
204 and 366
are equivalent to the UV chromatogram of the Con A
captured fraction of the HRP tryptic peptides, offering
further evidence that they are indeed glycopeptides.
The sensitivity of peaks in the ion chromatogram is
much higher than shown in the UV chromatogram. The
extracted trace of ion
m/z
163 can serve as a glycopeptide
diagnostic ion in a less sensitive way.
In this work, the SIM trace of ion
m/z
292 is a poor
match for the UV trace (data not shown). It is known that
oxonium ion 292 is from sialic acids (NeuAc+); therefore,
the observation that
m/z
292 is a poor diagnostic ion
for these experiments may indicate lack of sialic acid
containing oligosaccharide structures in the captured
HRP tryptic glycopeptides.
The cone voltage is critical because diagnostic ion peaks
under lower cone voltage, such as 100 V and 65 V in
mass spectrum, do not match the UV chromatogram.
Although
m/z
204 or 366 can be generated from
nonspecific fragmentation of peptide backbone,
simultaneous detection of both
m/z
204 and 366 provides
strong evidence that the peptide is glycosylated. When
comparing MS traces for
m/z
204 and 366 with the
UV chromatogram of the Con A captured fraction,
the peptides in the captured fraction can be identified
as glycopeptides.
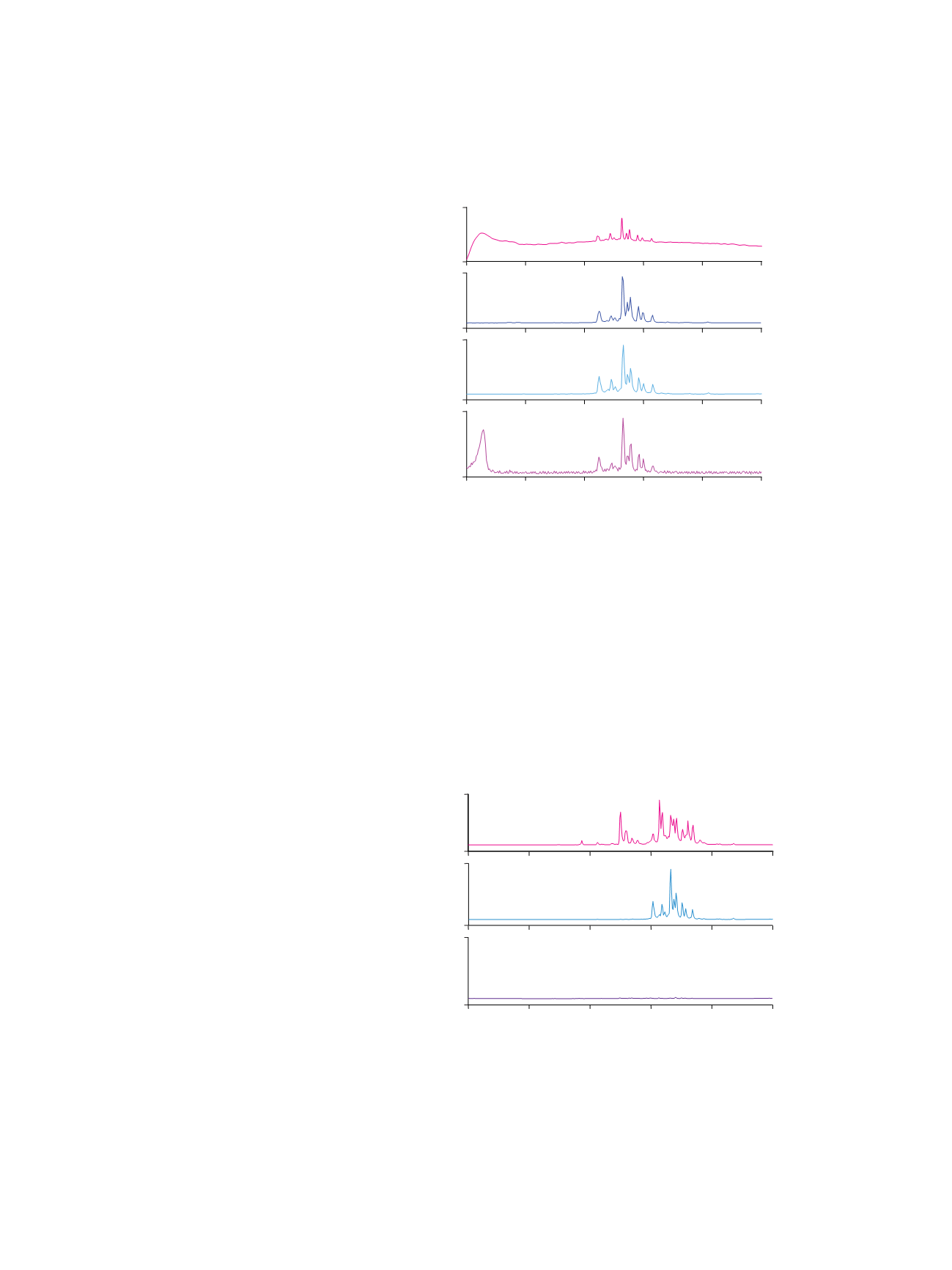
Figure 5 shows that scanning for diagnostic oxonium
ions is a selective and sensitive method to monitor
glycopeptides in a peptide mixture that has not been
passed through the ProSwift ConA-1S Affinity column.
Major peaks in the
m/z
204 SIM spectrum of unseparated
HRP tryptic digest fit with peaks in the spectrum of the
Con A captured fraction. Notice that some peaks shown in
HRP tryptic digest cannot be found in the Con A captured
fraction. These peaks may be lost in minor peaks (very
wide peaks such as peaks 3 and 4, shown in Figure 2,
with retention times of ~2.5 min and ~3.5 min) that elute
just after the flow-through peak. This fraction probably
has glycan structures that are not recognized by Con A.
Published literature shows that HRP does have a minor
glycan structure, Fuc(1-3)GlcNAc-, that could bind to
Con A, though very weakly.
5
Figure 4. Peptide mapping of Con A captured fraction from the HRP tryptic digest
detection by UV and MS in SIM mode.
0
14000
-20000
200000
-10000
100000
0
50
10
15
20
25
30
35
Minutes
Column:
Acclaim PA2, 3 µm (3.0 × 150 mm)
Mobile Phase:
A: Water with 0.05% formic acid
B: Acetonitrile with 0.04% formic acid
Gradient:
0–5.0 min, 0% B; 5–35.0 min,
0–50% B; 35.5–45.0 min, 90% B
Flow Rate:
0.425 mL/min
Inj. Volume:
20 µL
Temperature:
30 °C
Detection:
A
. UV at 214 nm
C
. m/z 204 in SIM mode
B
. m/z 366 in SIM mode
D
. m/z 163 in SIM mode
Sample Preparation: Tryptic peptides diluted with mobile phase A, 1 mg/mL solution
mAU
A
B
C
D
counts
counts
counts
-20000
200000
-20000
150000
-50000
400000
10
15
20
25
30
35
Minutes
Column:
Acclaim PA2, 3 µm (3.0 × 150 mm)
Mobile Phase: A: Water with 0.05% formic acid
B: Acetonitrile with 0.04% formic acid
Gradient:
0–5.0 min, 0% B; 5–35.0 min, 0–50% B; 35.5–45.0 min, 90% B
Flow Rate:
0.425 mL/min
Inj. Volume:
20 µL
Temperature:
30 °C
Detection:
m/z 204 in SIM mode
Samples:
A
. HRP tryptic peptides
B
. Con A captured fraction of HRP tryptic peptides
C
. Con A flow-through fraction of HRP tryptic peptides
A
B
C
counts
counts
counts
1
2
3
4
5
6
7
8
9 10
1112 13 14
1
2
4
3 5 6 7
8
9 10
1112 13 14
15 16 17
18
19
2021


