
Application Note 567
Key Words
Transcend TLX-1 System, TurboFlow Technology, Exactive Plus, Vitamin D
Goal
To demonstrate the effectiveness of a clinical research method for the
quantitation of 25-hydroxyvitamin D using online sample preparation
and high-resolution, accurate mass (HR/AM) quantitation with a Thermo
Scientific Exactive Plus Orbitrap mass spectrometer.
Analysis of 25-Hydroxyvitamin D in
Serum Using an Automated Online
Sample Preparation Technique with a
High-Resolution Benchtop Orbitrap Mass
Spectrometer
Matthew Berube, Joe DiBussolo, Catherine Lafontaine, Yang Shi
Thermo Fisher Scientific, Franklin, MA
Introduction
Blood levels of 25-hydroxyvitamin D
2
and 25-hydroxy-
vitamin D
3
are commonly tested by clinical researchers to
assess vitamin D sufficiency. In the last decade, liquid
chromatography coupled with triple quadrupole mass
spectrometry (LC-MS/MS) has become a popular
technique for such measurements. Due to their higher
resolving power relative to triple-stage quadrupole mass
spectrometers, Orbitrap™-based mass spectrometers are
better able to resolve analytes from sample matrices. In
addition, the ease of initial method set up and daily use
provides an advantage over triple-stage quadrupole mass
spectrometers for clinical research.
A method has been created that allows precipitated
serum to be injected into an HPLC system with minimal
sample preparation and analyzed by an Exactive
TM
Plus
benchtop Orbitrap mass spectrometer. Total method time
is 7.75 minutes on a Thermo Scientific Transcend TLX-1
system utilizing TurboFlow technology. Throughput can
be increased to a sample every 3.7 minutes by using a
Transcend™ TLX-2 multiplexed UHPLC system or
1.9 minutes with a Transcend TLX-4 system.
Experimental
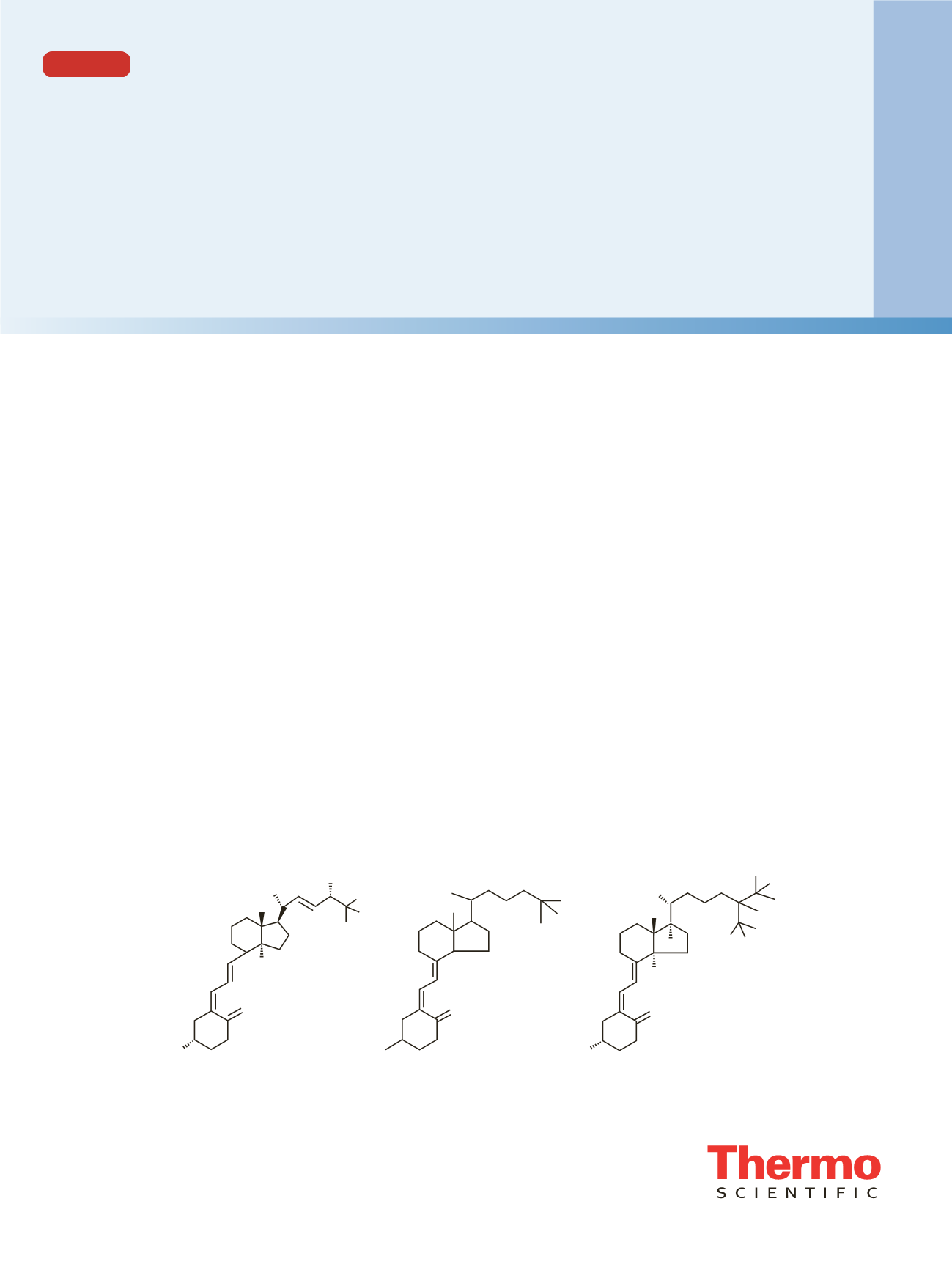
Standard solutions of 25-hydroxyvitamin D
2
, 25-hydroxy-
vitamin D
3
, and deuterated 25-hydroxyvitamin D
3
internal
standard were obtained from Cerilliant, Inc. (Figure 1).
Six calibrators at 2, 5, 10, 25, 50 and 100 ng/mL and
three QCs at 5, 40 and 80 ng/mL were prepared by
fortifying bovine serum albumin diluent with 200 ng/mL
25-hydryoxyvitamin D
2
and D
3
standard mix. Precipitating
reagent was prepared by adding deuterated D
6
-25-hydroxy-
vitamin D
3
to acetonitrile for a final concentration of
75 ng/mL. In addition, pooled human serum samples were
crashed 2 to 1 with acetonitrile and spiked with analytes
for a final concentration of 20 ng/mL for 25-hydroxy-
vitamin D
2
and 25-hydroxyvitamin D
3
, and 50 ng/mL
of D
6
25-hydroxyvitamin D
3
internal standard.
Figure 1. Analytes
CH
2
HO
H
H
3
C
CH
3
D
OH
D
D
D
D
D
H
H
3
C
CH
3
CH
3
CH
3
OH
CH
3
CH
2
HO
H
3
C
CH
3
CH
3
CH
3
OH
HO
CH
2
25-Hydroxyvitamin D
2
C
28
H
44
O
2
(MW 412.65)
25-Hydroxyvitamin D
3
C
27
H
44
O
2
(MW 400.63)
D
6
-25-Hydroxyvitamin D
3
C
27
H
38
D
6
O
2
(MW 406.67)
H



















