
3
System eluents (Fisher Chemical
™
brand) were as follows:
Loading and Eluting Pumps A:
0.1 % (v/v) aqueous formic acid
Loading and Eluting Pumps B:
0.1 % (v/v) formic acid in methanol
Loading Pump C:
Acetone/2-propanol/acetonitrile
(1:2:2 v/v/v)
Liquid Chromatography
Sample supernatants (100 µL) were injected onto a
TurboFlow XL C18 column (50 x 0.5 mm i.d.) under
turbulent flow conditions (2 mL/min). Retained analytes
were back-flushed from the TurboFlow column using
elution solvent stored in a holding loop and focused onto
a Thermo Scientific
™
Accucore
™
PFP analytical column
(2.6 µm particle size, 50 x 2.1 mm i.d.) maintained at
40 °C. During isocratic elution (0.40 mL/min) from the
analytical column, the TurboFlow column was back-
flushed with eluent C and the elution solvent loop refilled.
Eluent flow was diverted to waste for 8 minutes following
each injection onto the TurboFlow columns. The system
was then re-equilibrated prior to the next injection.
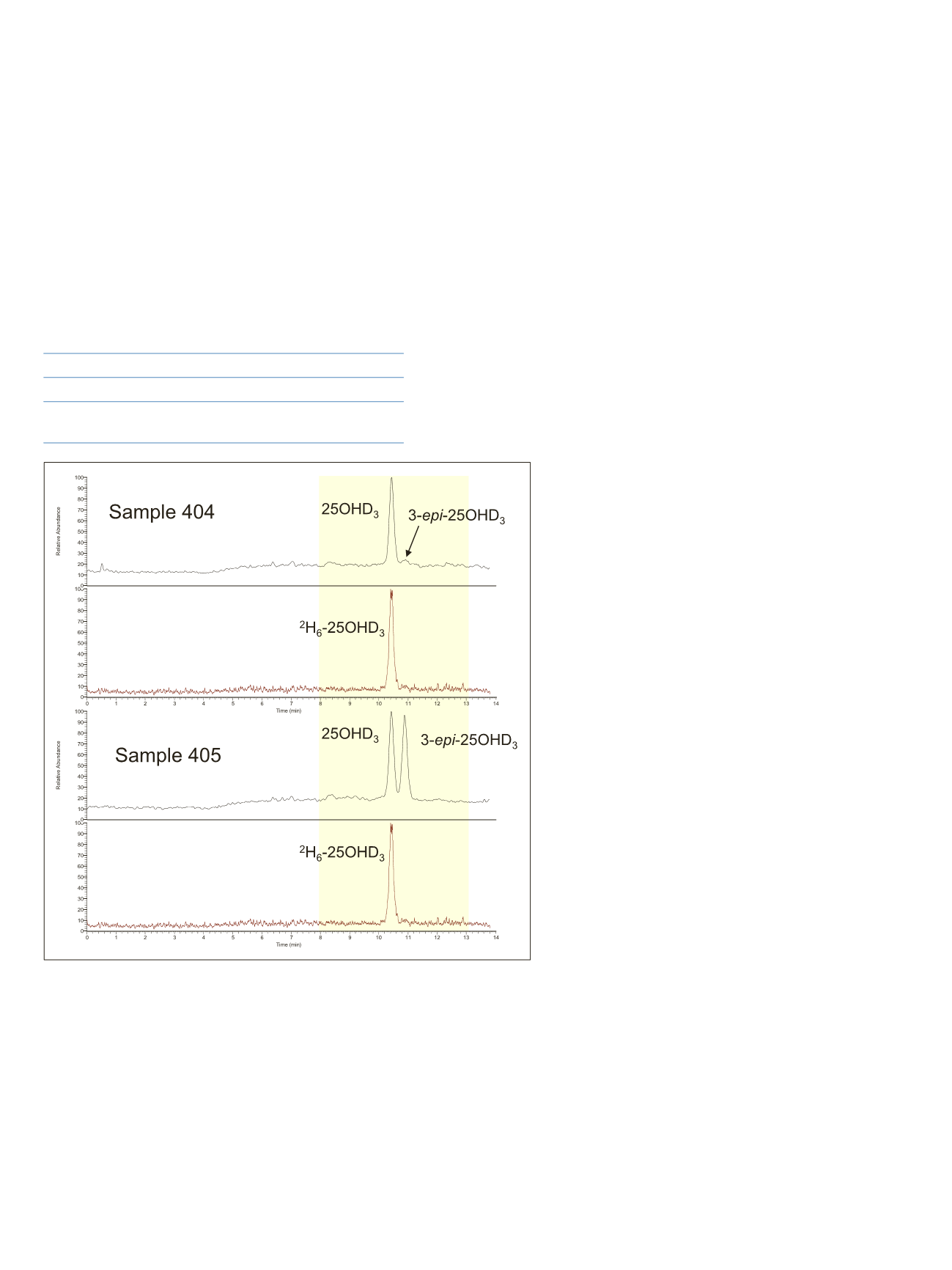
Figure 7. Chromatograms showing resolution of 3-
epi
-25OHD
3
from 25OHD
3
in DEQAS
samples 404 and 405. 25OHD
2
chromatograms not shown. Sample 405 was prepared by
addition of 3-
epi
-25OHD
3
to sample 404.
4
Yellow highlighted area corresponds to data
window for multiplexing.
Mass Spectrometry
Mass spectrometry was carried out in positive ionization
mode using atmospheric pressure chemical ionization
(APCI) on a Thermo Scientific
™
TSQ Vantage
™
triple-stage
quadrupole LC-MS/MS system. Selected-reaction
monitoring (SRM) transitions did not include water-loss
fragmentations.
3
MS/MS data were acquired for 5 minutes
per analysis to allow multiplexing. Total LC time was
14 minutes, as shown in Figure 7, which means the
TLX-2 system could do one analysis every 7 minutes).
Results and Discussion
As shown in Figure 7, retention times were 10.94, 11.47,
and 11.82 minutes for 25OHD
3
, 3-
epi
-25OHD
3
, and
25OHD
2
, respectively. Total analysis time was 14 minutes,
including column re-equilibration. Sample 405 was correctly
found to contain 3-
epi
-25OHD
3
. This compound would
have been misidentified as additional 25OHD
3
if a C18
analytical column, which does not resolve the epimer well,
if at all, had been used as part of the LC-MS/MS method.



















