
Automated, High-Throughput
LC-MS/MS Workflow for the Analysis
of 25-Hydroxyvitamin D
2/3
and
3-
epi
-25-Hydroxyvitamin D
3
Lewis Couchman
1
, Caje Moniz
1
, Sarah Robinson
2
, Justin Brooking
2
1
Department of Clinical Biochemistry, King’s College Hospital - NHS Foundation Trust, London, UK
2
Thermo Fisher Scientific, Hemel Hempstead, UK
Application Note
584
Key Words
Vitamin D, 25-hydroxyvitamin D
2/3
, 3-
epi
-25-hydroxyvitamin D
3
, TurboFlow
method, Transcend, TSQ Vantage, Versette, LC-MS/MS
Goal
Develop an automated, high-throughput LC-MS/MS workflow for the
analysis of 25-hydroxyvitamin D
2/3
and 3-
epi
-25-hydroxyvitamin D
3
in
human serum for research laboratories.
Introduction
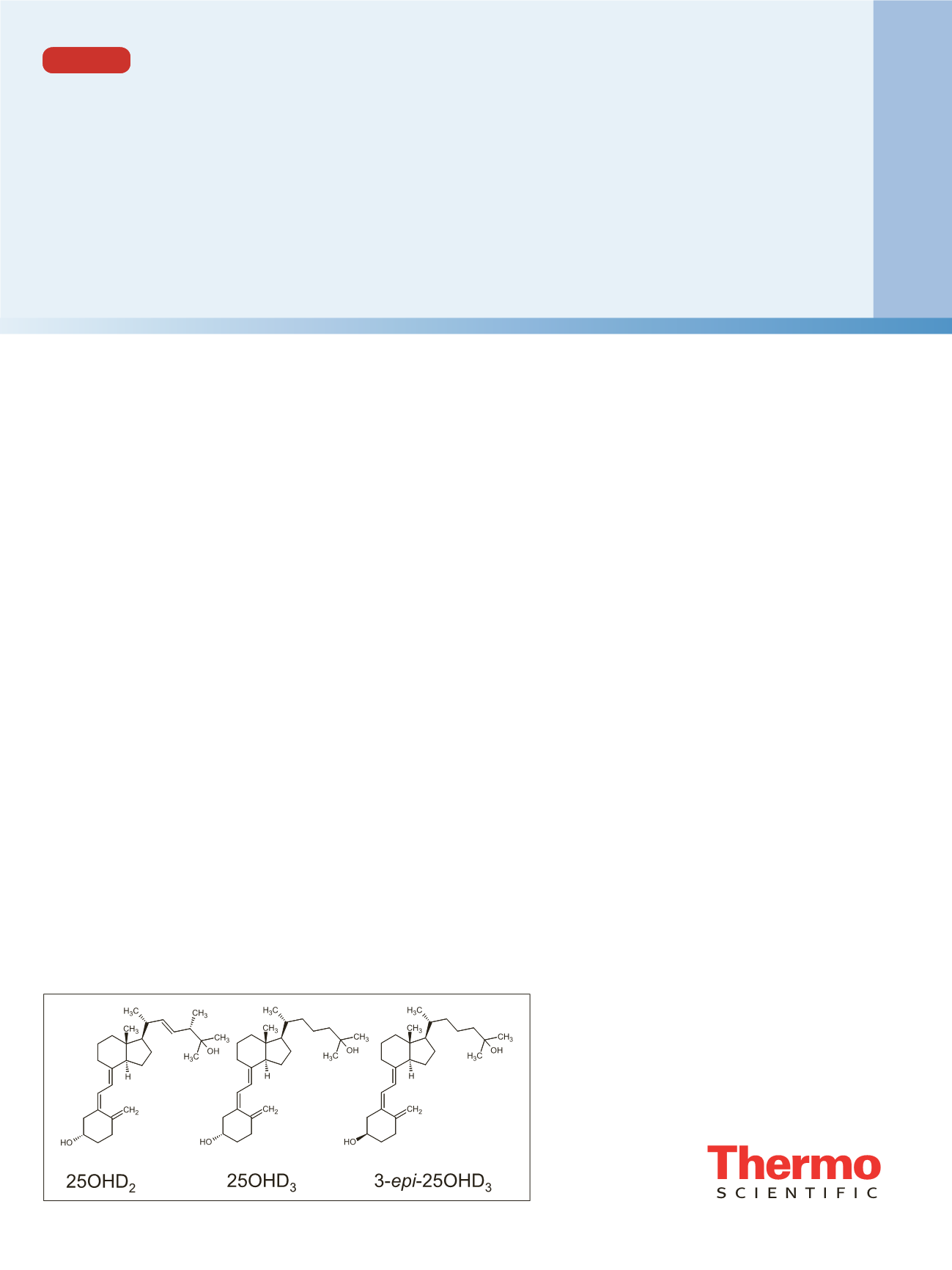
Analysis of total serum 25-hydroxyvitamin D
2
/D
3
(25OHD,
shown in Figure 1) is performed routinely in many
research laboratories. Demand for this analysis continues
to grow, and liquid chromatography with tandem mass
spectrometry (LC-MS/MS) is increasingly used for this
purpose.
1
Compared with other sample preparation techniques,
Thermo Scientific
™
TurboFlow
™
technology has been
shown to significantly improve the removal of matrix
components prior to LC-MS/MS analysis of 25OHD.
2
However, there are additional pre-analytical steps that
must be performed, the most important of which are the
complete removal of the analytes from the endogenous
vitamin D binding protein and the addition of
isotopically-labeled internal standards for quantitation.
When performed manually, these steps can increase the
total analysis time by approximately two hours for a
batch of 96 samples. This application note presents a
workflow that uses an automated liquid handling system
to reduce the time required to prepare a 96 well plate for
analysis to less than 20 minutes.
Further, MS/MS alone is an achiral technique. This can be
problematic for some isobaric 25OHD metabolites,
notably 3-
epi
-25-hydroxyvitamin D
3
(shown in Figure 1
as 3-
epi
-25OHD
3
). For accurate LC-MS/MS analysis of
25OHD
3
, LC-MS/MS extended chromatographic analysis
times are needed to resolve 3-
epi
-25OHD
3
. In this
application note, multiplexing technology is used to
maximize throughput of the chromatographic method
used to resolve interfering 3-
epi
-25OHD
3
.
Experimental
Sample Preparation
Human serum samples from the international Vitamin D
External Quality Assessment Scheme (DEQAS, samples
404 and 405) were used for the analysis.
All liquid handling was carried out using a Thermo Scientific
™
Versette
™
automated liquid handling system. An overview
of the liquid handling procedure is shown in Figure 2.
The Versette system was fitted with a 96 channel pipetting
head and Thermo Scientific
™
D.A.R.T.’S
™
300 µL extended-
tip disposable pipette tips. Calibration standards and
quality controls (both from the Chromsystems MassChrom
®
25-hydroxyvitamin D
2/3
kit) and samples (100 µL) were
transferred from decapped 1 mL Thermo Scientific
™
Nunc
™
Cryobank storage vials to a 96 well filter plate.
Internal standard solution (25 µL,
2
H
6
-25OHD
3
) and
precipitation reagent (200 µL), both from Chromsystems,
were then added separately from the reagent reservoirs.
The filter plates were covered and mixed on a plate shaker
(600 rpm, 10 min). Supernatents were collected into a
microtitre plate by centrifugation (200
g
, 3 min). The
plate was sealed with an adhesive plate seal and transferred
to a Thermo Scientific
™
Transcend
™
TLX-2 system for
analysis. All of the consumables utilized in the process are
listed in Table 1.
Figure 1. Structures of 25-hydroxyvitamin D
2
/D
3



















