
2
Experimental
Approach
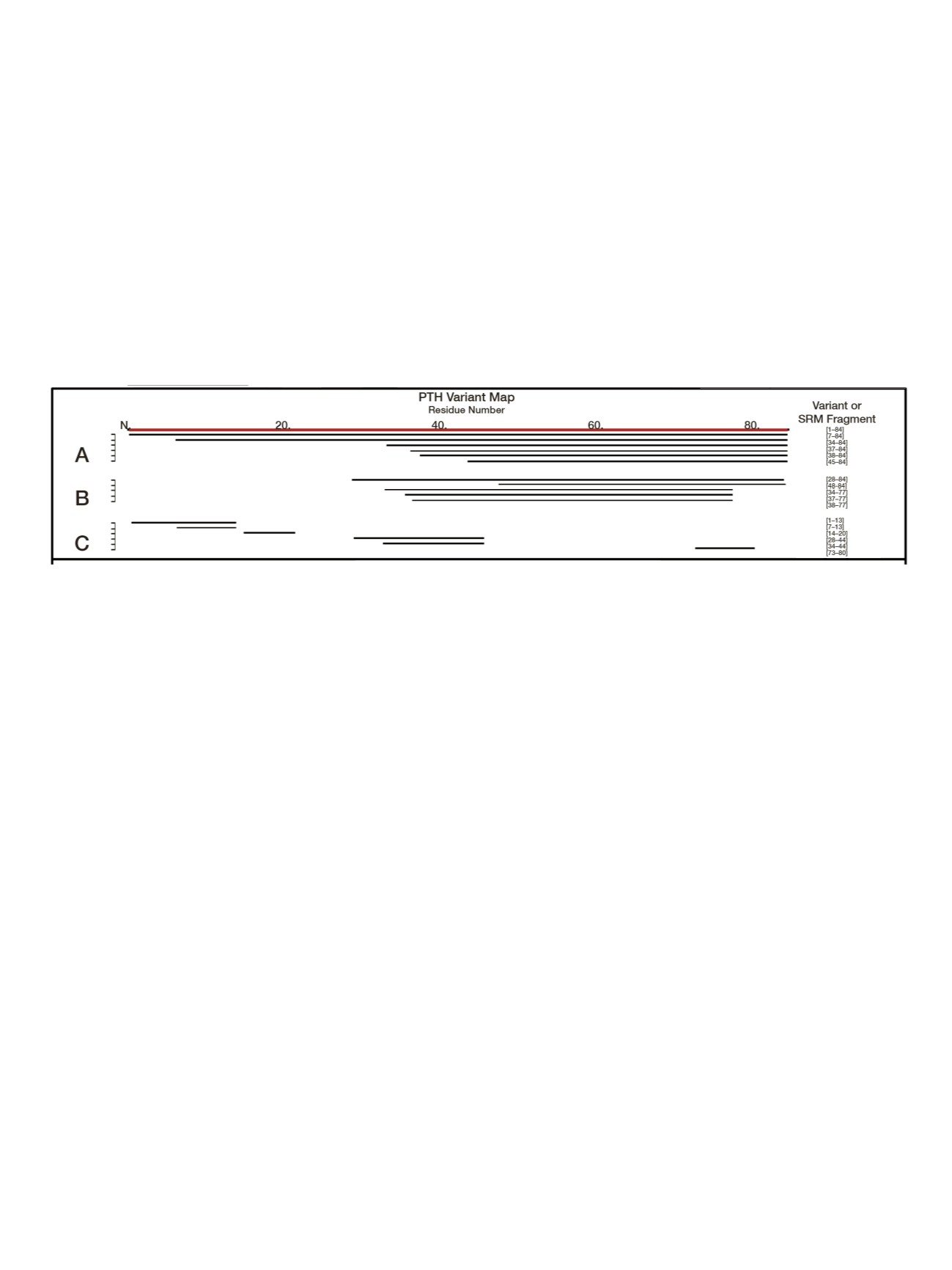
In addition to the well-characterized truncated PTH
variants, PTH1–84 and PTH7–84, four other molecular
versions have been reported in the literature as present in
human biofluids (primarily plasma or serum). Aligning these
fragments to the sequence of PTH1–84 produced a variant
map revealing forms stemming predominantly from
N-terminal truncations (Figure 1). A conserved region
(among several variants) was evident between residues 48
and 84. This region was suitable for immunoaffinity
targeting to capture ragged N-terminal variants (for
example, PTH1–84 and PTH7–84). Postcapture digestion
of retained PTH (and variants) created the basis for
SRM-MSIA,
8-11
for which surrogate peptides representative
of the different PTH variants were selected for analysis.
Reagents
Goat polyclonal anti-PTH39–84 antibody was purchased
from Immutopics International. Recombinant human PTH
(rhPTH) was obtained from Bachem. Premade 0.01 M
HEPES-buffered saline with 3 mM EDTA and 0.05%
(vol/vol) surfactant P20 (HBS-EP) was purchased from
Biacore. Thermo Scientific
™
Pierce
™
premixed
2-[morpholino]ethanesulfonic acid–buffered saline powder
packets and Thermo Scientific synthetic heavy-labeled
peptides were used. High purity solvents from Fisher
Chemical brand were used.
Samples
A total of 24 plasma samples were used in the research
study: 12 from individuals with previously diagnosed severe
renal impairment or end-stage renal disease (ten males and
two females; mean age 66.7 years) and 12 from healthy
individuals (ten males and two females; mean age 65 years).
Among the individuals with renal failure, three were
Hispanic, two were Asian, two were African American, and
six were Caucasian. The ethnicity information for the
healthy sample donors was not available.
Calibration Curves Samples
Samples for creation of calibration curves were prepared
from pooled human plasma by step-wise, 2-fold serial
dilution of an initial sample containing rhPTH at a
concentration of 1000 ng/L (eight steps, range
1000–7.8 ng/L). Samples were frozen at -80 °C until use.
Sample Preparation and Immunocapture
Purification and concentration of the PTH was accomplished
by immunoaffinity capture. Extraction of PTH from plasma
was carried out with proprietary Thermo Scientific
™
Mass
Spectrometric Immunoassay (MSIA
™
) pipette tips derivatized
with the PTH antibodies via 1,1′-carbonyldiimidazole
chemistry.
13-17
After extraction, PTH was digested, separated
by liquid chromatography, and analyzed by high-resolution
MS/MS on an ion trap-Orbitrap
™
hybrid mass spectrometer
and by SRM on a triple quadrupole mass spectrometer as
described below.
Sample Elution and Trypsin Digestion
Bound proteins were eluted from the tips into a 96 well
plate by pipetting 100 µL of 30% acetonitrile/0.5% formic
acid up and down for a total of 15 cycles. Samples were
lyophilized to dryness and then resuspended in 30 µL of
30% n-propanol/100 mmol/L ammonium bicarbonate,
pH 8.0, diluted with 100 µL of 25 M acetic acid containing
100 ng of trypsin. Samples were allowed to digest for 4
hours at 37 °C. After digestion, samples were lyophilized
and resuspended in 30 µL of 3% (vol/vol) acetonitrile/0.2%
(vol/vol) formic acid/glucagon/PTH heavy peptides.
Figure 1. PTH variant map. (A) N-terminally truncated PTH variants identified previously.
7,12
(B) Variants added to map by top-down MS analysis.
(C) Conserved and truncated tryptic fragments chosen for SRM-MSIA.



















