2
Sheathless Capillary Electrophoresis Mass Spectrometry (CESI-MS) as a Versatile and Powerful Tool for the Characterization of Monoclonal Antibodies
Overview
Purpose:
To assess the performance of sheathless capillary electrophoresis
electrospray ionization (CESI) coupled to a hybrid quadrupole-Orbitrap mass
spectrometer for the characterization of tryptic digests of monoclonal antibodies
Methods:
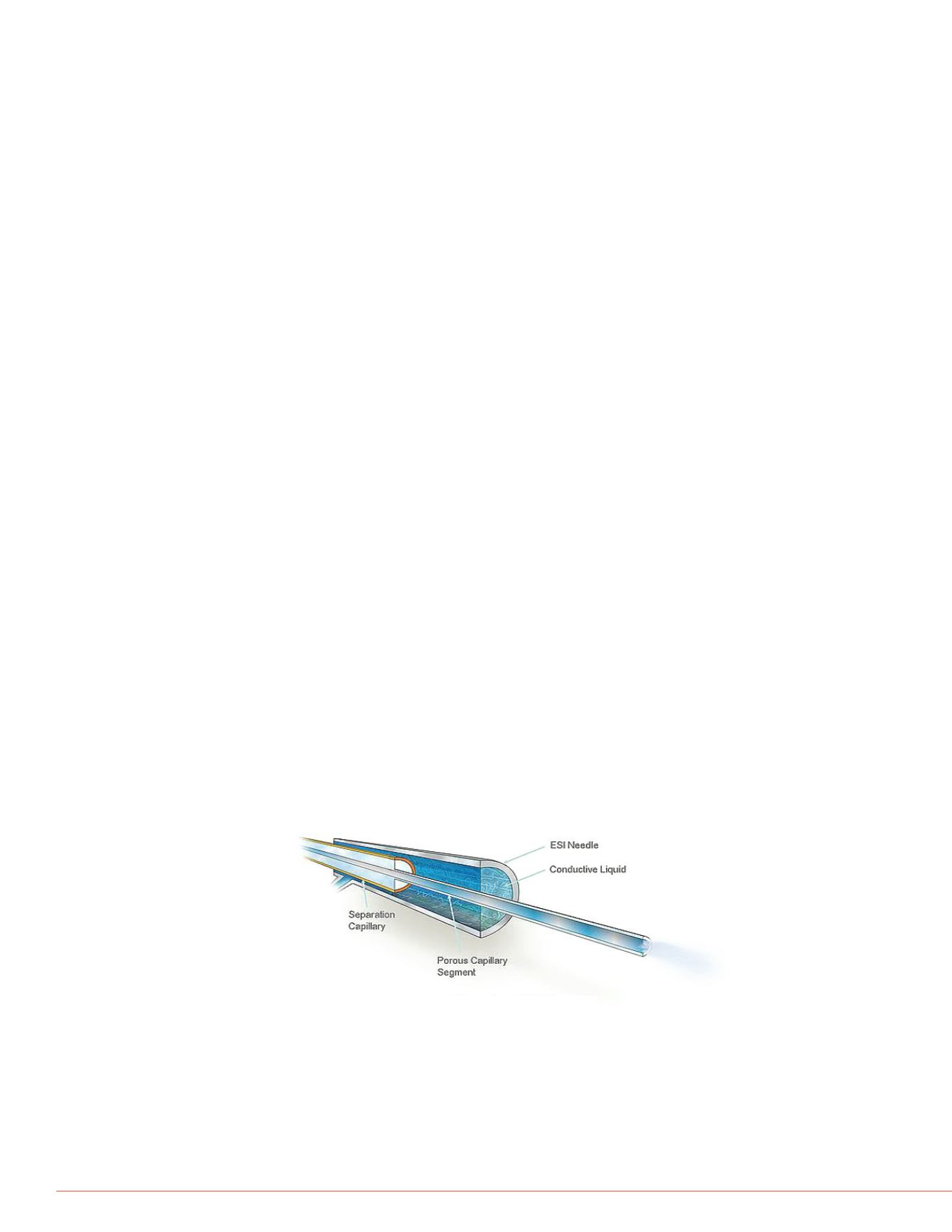
CESI-MS - sheathless hyphenation of capillary electrophoresis (CE) with
electrospray ionization mass spectrometry (ESI-MS) through the use of a separation
capillary with a porous tip
Results:
100% sequence coverage & in-depth characterization of glycosylations in a
single CESI-MS run
Introduction
In the last decade, the percentage of therapeutics based on monoclonal antibodies
(mAbs) have been growing significantly. The number of molecules currently in clinical
trials (innovators and/or biosimilars) indicate that the slope is becoming even steeper.
Characterization of mAbs is important as unpredicted impurity or heterogeneity can
impact therapeutic safety and/or efficacy. mAbs are large glycosylated
molecules (≈
150 kDa), containing several other post-translational modifications (PTMs). As a result,
the analytical characterization of mAbs is usually complex and cumbersome. Aiming at
improving the capabilities of the analytical toolbox available for mAb characterization, a
novel platform combining sheathless capillary electrophoresis with fast, highly resolving
and accurate mass (HRAM) mass spectrometry, has been assessed for primary
sequence and glycosylation characterization of mAbs. Using two different molecules,
Trastuzumab and Bevacizumab, the capabilities of the sheathless CESI-MS platform
have been evaluated. Initially focusing on peptide mapping, the versatility of the
platform has been demonstrated. It has been shown that the platform was capable of
separating and unambiguously identifying, within a single run, very different molecules
ranging from small dipeptides to very large peptides (Mw> 8000 Da). Taking advantage
of the good compatibility between the speed of CESI and the duty cycle capabilities of
the mass spectrometer in MS/MS mode, 100% sequence coverage has been obtained
for the two tested molecules. As glycosylation represents such an important attribute of
mAbs, the capabilities of the platform for the study of glycoforms was further assessed.
In this context, it was found that the sensitivity of the platform was an enabling
parameter.
Methods
Samples were prepared following a classical but short (2-hour digestion) protocol
involving DTT, iodoacetamide, RapiGest and trypsin. CESI experiments were carried
out with a Beckman Coulter™ CESI prototype system equipped with a temperature
controlled autosampler and a power supply with the ability to deliver up to 30 kV.
Prototype fused-silica capillaries with porous tip were used
.
Solutions of 10% acetic
acid and ammonium acetate (pH 4 and various ionic strengths) were employed as
background electrolyte (BGE) and leading electrolyte, respectively. CESI separations
were performed at 20 kV,
and about 100 fmol (45 nL of a 2 mM solution) of each tryptic
digest were injected per analysis. Eluted peptides were analyzed using a Thermo
Scientific™ Q Exactive™ bench
-top quadrupole Orbitrap mass spectrometer with a
data-dependent top ten HCD method. High resolution HCD spectra were analyzed
using Thermo Scientific™ Proteome Discoverer™ software version 1.3.
Schematic of CESI Sprayer
130115_cem
RT:
15.05 - 5
1
0
20
40
60
80
100
0
20
40
60
80
100
Relative Abundance
0
20
40
60
80
100
130115_cem
RT:
15.04 - 5
1
0
20
40
60
80
100
0
20
40
60
80
100
Relative Abundance
0
20
40
60
80
100
16.
1
FIGURE
FIGURE
FIGURE
RT:
20.03 -50.05
0
20
40
60
80
100
0
20
40
60
80
100
0
20
40
60
80
100
RelativeAbundance
0
20
40
60
80
100
0
20
40
60
80
100
21.08
21.
20.65
Capillary Electrophoresis Mass Spectrometry (CESI-MS) as a
iqi Hao
2
, David Horn
2
, Alain Beck
3
, Patrick Bennett
2
, Jean-Marc Busnel
1
lter Inc., Brea, CA, USA;
2
Thermo Fisher Scientific Inc., San Jose, CA,
abre, Saint-Julien, France
with
tion
in a
130115_cems_beva-20kv5psi60sec60mi_06
1/16/2013 12:45:49 AM
RT:
15.04 - 55.08
60
80
100
29.11
28.16
31.48
27.95
25.75
22.15
NL: 3.70E10
TIC F: FTMS + p NSI
Full ms
[150.00-1800.00] MS
130115_CEMS_Beva-
20kv5psi60sec60mi_0
100% Sequence Coverage with CESI-MS
Bevacizumab and Trastuzumab were initially considered to assess the capabilities of
CESI-MS for achieving high sequence coverage of mAbs. Each molecule was digested
for 2 hours with trypsin and the obtained peptide mixture was analyzed directly after by
CESI-MS.
FIGURE 1. Triplicate analysis of a tryptic digest of Bevacizumab
FI


