5
Thermo Scientific Poster Note
•
PN ASMS13_WP24_ELewis_e 06/13S
Conclusion
An automated workflow w
which combined online tr
A trypsin column was dev
elevated temperatures.
Operation under denaturi
pretreatment.
Direct coupling of this sys
Orbitrap mass spectrome
for the monoclonal antibo
A confident peptide mapp
Orbitrap LCMS/MS analy
Potential future applicatio
monitoring and disulfide b
References
1. Götze,et al. J. Am. Soc.
2.
2. Vermeer et. al. Biophysic
3. Kumakura et. al. Journal
FIGURE 7: Base peak chromat
and alkylation.
best-case scenario trypsin that
of the activity 30 minutes at 70 C.
cess of the enzymes three
under these harsh conditions.
Perfinity is a trademark of Perfinity Biosci
trademarks are the property of Thermo Fi
This information is not intended to encour
intellectual property rights of others.
Table 2. Coverage determinations of mAb digests.
RT:
0.00 -30.02
0
2
4
6
8
10
0
5
10
15
20
25
30
35
40
45
50
55
60
65
70
75
80
85
90
95
100
RelativeAbundance
8.21
575.80
9.08
547.83
4.95
395.65
7.15
464.70
9.17
501.28
3.49
441.13
10.5
490.5
1.96
538.26
0.63
515.64
RT:
0.00 -19.93
0
1
2
3
4
5
6
7
8
9 10 11 12 13 14 15 16 17 18 19
Time (min)
0
5
10
15
20
25
30
35
40
45
50
55
60
65
70
75
80
85
90
95
100
RelativeAbundance
11.88
634.75 12.82
693.24
16.44
1273.71 17.58
971.42
14.20
730.08
17.61
971.44
8.85
490.57
6.90
524.55
8.30
651.89
12.88
693.30 14.25
773.50
3.86
390.97
8.88
490.59
14.30
773.48
11.41
742.58
6.57
531.81
9.27
748.62
6.98
524.61
10.32
626.72
16.05
1122.28
3.92
391.05
7.58
694.55
3.84
391.03
15.67
1052.34
5.28
367.28
2.91
415.98
17.68
971.35
5.30
550.14
1.68
446.86
1.57
377.77
2.78
349.29
17.73
971.08
19.56
742.95
NL:
1.28E8
BasePeak
MS
r&ahuiggin10%
bc_10000ngml
_r1_topten_12j
uly2012
FIGURE 5: Base peak chromatogram of native mAb digestion without reduction
and alkylation.
TABLE 6. Coverage determinations of native HSA digests.
e antibody samples were performed
ease in efficiency was observed at
itions the samples were
ted. This observation validates
t the denaturation of antibody
n at 70 C (3).
s and temperatures.
As shown in Table 2, digestion of reduced and alkylated mAb samples yielded
sequences coverage exceeding 90%. Even for the samples of digested without
pretreatment sequence coverage was 79% for heavy chain and 97% for light chain. The
base peak chromatograms obtained for native antibody digestion is shown in Figure 5.
This online digestion experiment was also applied to native human serum albumin. The
extensive disulfide linkages in this molecule makes it an especially challenging case for
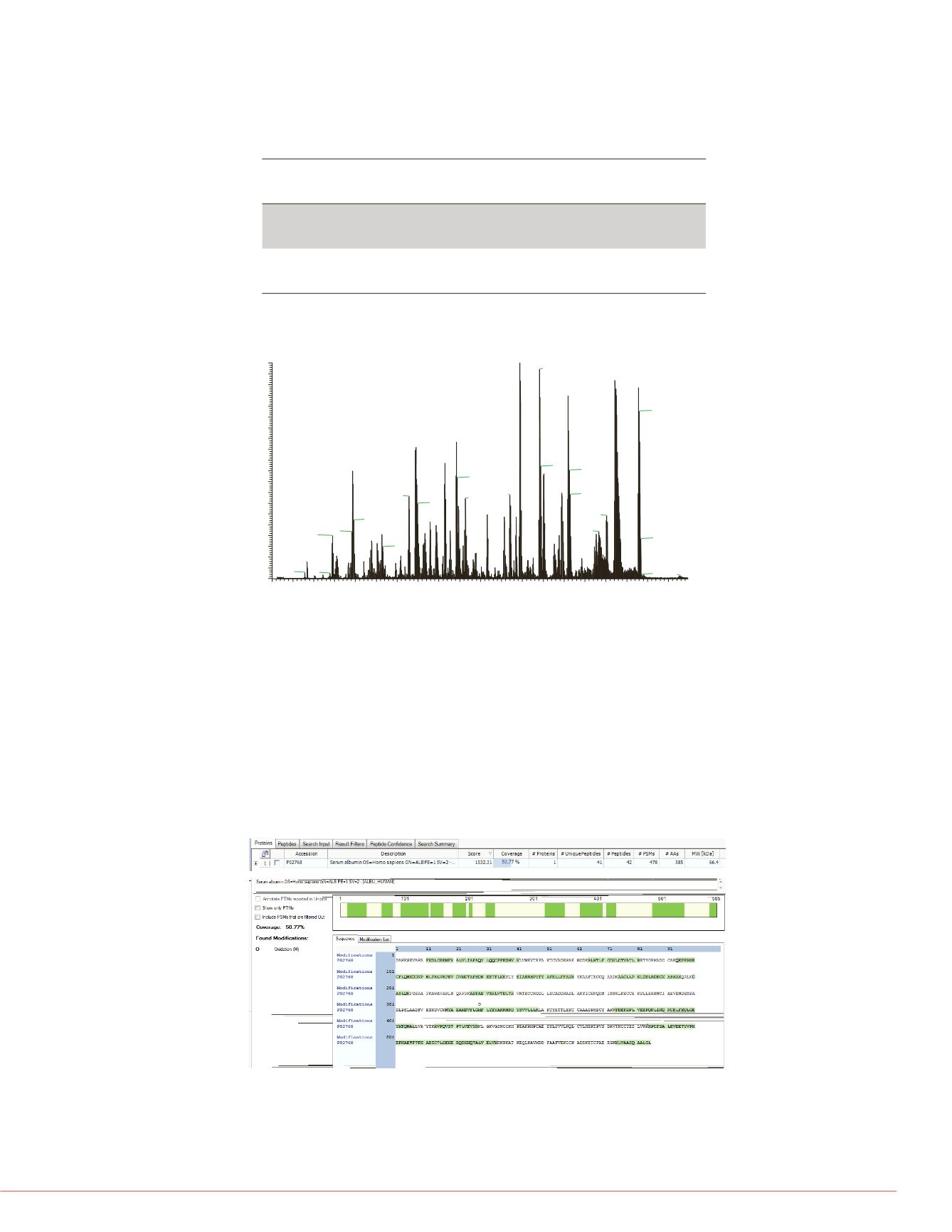
native digestion. Figure 6 shows the coverage determinations of native HSA digests.
Figure 7 shows the base peak chromatogram of native HSA digestion without reduction
and alkylation. The identification of a large number of canonical peptides suggests native
human serum albumin (HSA) was effectively digested. Given the highly bridged nature of
HSA this data suggests that many different types of less stable proteins and antibodies
can effectively be digested under these conditions.
Since the total digest time for this work was <5 minutes it is possible that this workflow
would be extremely useful in situations where close to real time monitoring would be
advantageous such as process monitoring. Because reduction and alkylation are not
necessary, it is possible that this technology could also be successfully applied to disulfide
bond mapping.
Sample
Light Chain
Heavy chain
Reduced and Alkylated
100%
91%
Native
97%
79%
was developed capable of
mperatures.


