
Part of Thermo Fisher Scientific
For Research Use Only. Not for use in diagnostic procedures.
www.thermoscientific.comLegal Notices: ©2011 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. This
information is presented as an example of the capabilities of Thermo Fisher Scientific Inc. products. It is not intended to encourage use of these products in any
manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change. Not all products are available in all
countries. Please consult your local sales representative for details.
Thermo Fisher Scientific,
San Jose, CA USA is ISO Certified.
AN63465_E 09/11S
In addition to these
offices, Thermo Fisher
Scientific maintains
a network of represen
tative organizations
throughout the world.
Africa-Other
+27 11 570 1840
Australia
+61 3 9757 4300
Austria
+43 1 333 50 34 0
Belgium
+32 53 73 42 41
Canada
+1 800 530 8447
China
+86 10 8419 3588
Denmark
+45 70 23 62 60
Europe-Other
+43 1 333 50 34 0
Finland/Norway/
Sweden
+46 8 556 468 00
France
+33 1 60 92 48 00
Germany
+49 6103 408 1014
India
+91 22 6742 9434
Italy
+39 02 950 591
Japan
+81 45 453 9100
Latin America
+1 561 688 8700
Middle East
+43 1 333 50 34 0
Netherlands
+31 76 579 55 55
New Zealand
+64 9 980 6700
Russia/CIS
+43 1 333 50 34 0
South Africa
+27 11 570 1840
Spain
+34 914 845 965
Switzerland
+41 61 716 77 00
UK
+44 1442 233555
USA
+1 800 532 4752
Conclusion
A sensitive LC-MS/MS method was developed to quantify
plasma free metanephrines in clinical research laboratories.
This method has an LLOQ of 7.2 and 18.0 pg/mL for
metanephrine and normetanephrine, respectively. Method
precision ranged from 2.0% to 10.9%. Ion-pairing
SPE was used for sample preparation, and a Hypercarb
column was used for chromatographic separation of
metanephrines.
Reference
1. He, X.; Gabler, J.; Yuan, C.; Wang, S.; Shi, Y.; Kozak, M. Quantitative
Measurement of Plasma Free Metanephrines by Ion-pairing Solid Phase
Extraction and Liquid Chromatography-Tandem Mass Spectrometry with
Porous Graphitic Carbon Column
J. Chromatogr. B Analyt. Technol.
Biomed. Life Sci.
2011
, 879(23), 2355-2359.
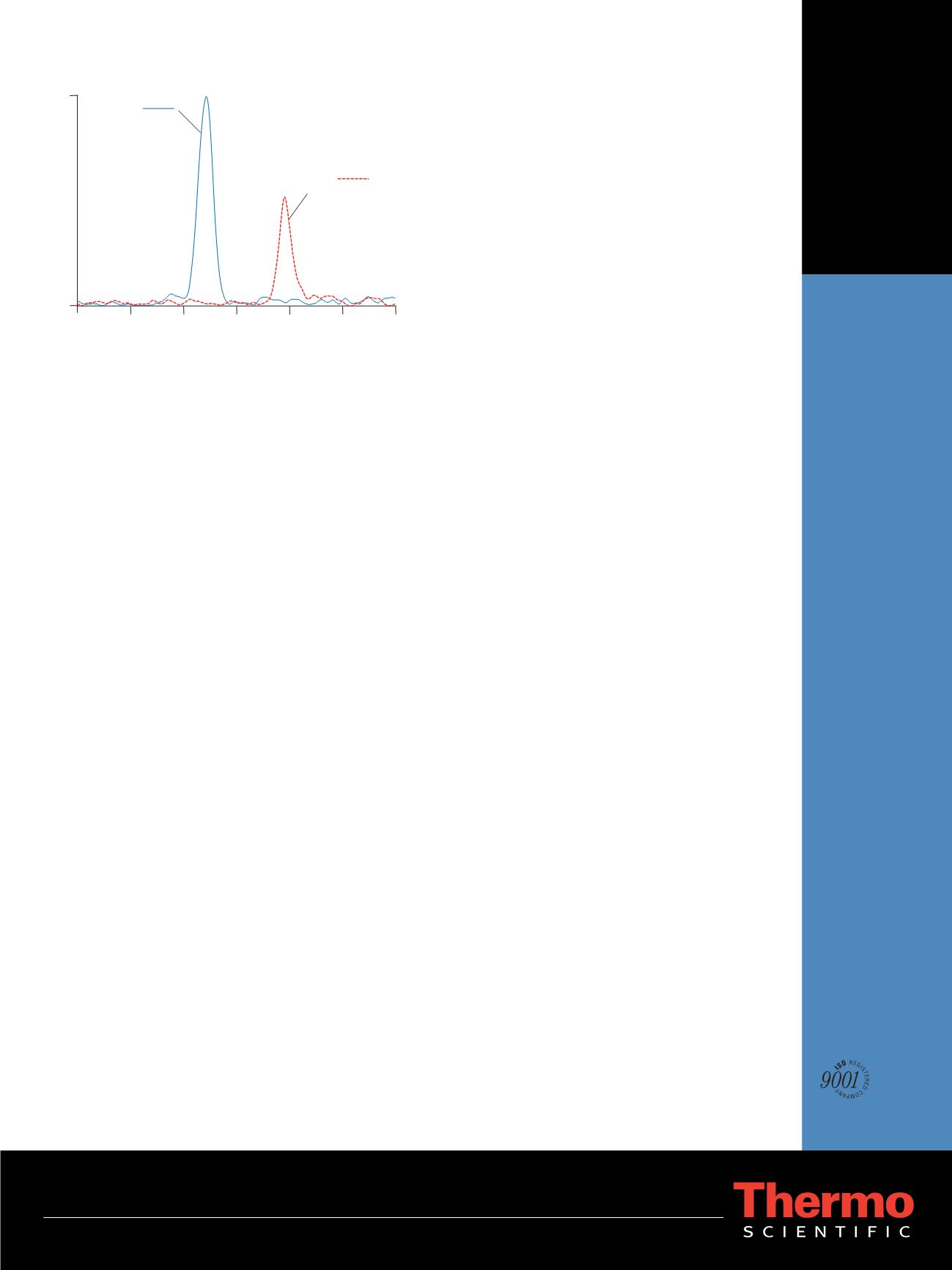
MN:
m/z
180 148
20 pg/mL
NMN:
m/z
166 134
104 pg/mL
Retention Time (min)
2.8
2.95
3.1
3.25
3.4
3.55 3.7
0
1960
Intensity
Figure 6. Representative SRM chromatograms of MN and NMN using a
processed human plasma sample



















