3
Thermo Scientific Poster Note
•
PN70513_E_04/14S
id chromatography (HPLC)
PLC)
columns for high-
f native and fluorescently
uding antibodies.
or the chromatographic analysis
) and LC-MS/MS analysis for
-glycans from proteins by MS
ca-based HPLC/UHPLC column
esigned for simultaneous
designed for high-resolution,
r biologically relevant glycans,
ds.
‘free state’ and conjugated
cans. They are involved in a
The functions, including
ombinant proteins and
n the structure and types of
cans are quite diverse,
modifications and physiological
tive estimation of glycans is
ical projects.
3
However, it is
erize glycan profiles and
HPLC/UHPLC columns
ntitative analysis of glycans.
hput analysis, with unique
lycans from antibodies
—
either
ods. Because glycans are
teraction liquid chromatography
packing materials are often
te glycans mainly by hydrogen
aration. However, identification
pes of columns because
he separation envelope, making
which is based on advanced
these limitations and can
onfiguration. In addition, each
AXH-1 column provides both
ter quantitative analysis.
se F enzyme. Conjugate the
group using the reported
11), 2-AB A2 (P/N GKSB 312),
rozyme
®
(Hayward, CA). Prior
ammonium formate, pH = 4.4)
and 70% acetonitrile.
ific
™
Dionex
™
UltiMate
™
3000
™
UltiMate
™
r or MS detector.
Q Exactive
™
Benchtop
gs: MS scan range 380
–
2000.
ith AGC target of 1e
6
; and DDA
C target of 2e
5
.
r glycan identification and
re accepts raw data files from
s the associated glycan
es.
Results
Separation of Labeled Glycans Based on Charge, Size, and Polarity
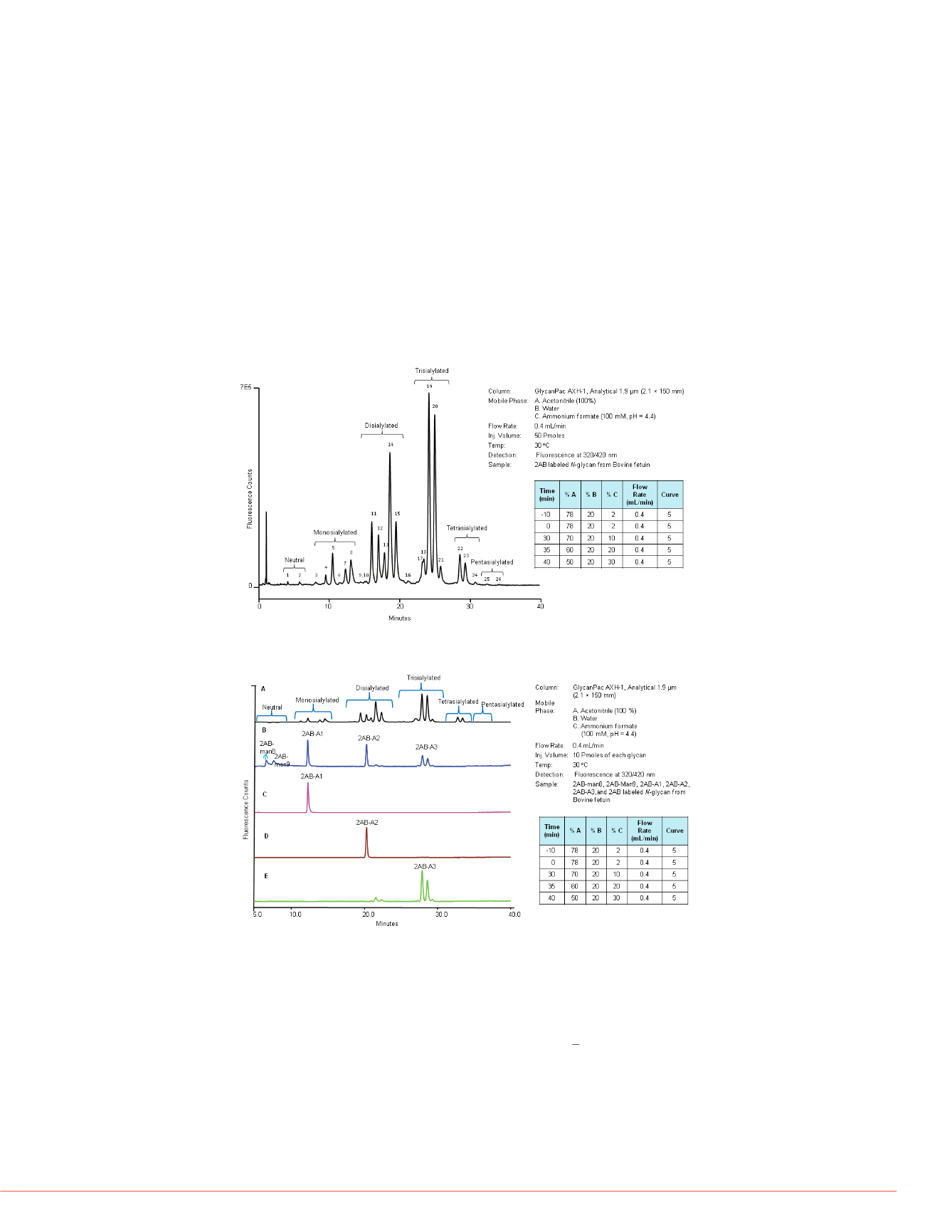
The GlycanPac AXH-1 column can be used for qualitative, quantitative, and structural
analysis as well as characterization of uncharged (neutral) and charged glycans present
in proteins. Figure 1 shows bovine fetuin on the GlycanPac AXH-1 (1.9
μ
m, 2.1
×
150 mm)
column using fluorescence detection. The separation and elution of glycans are based on
charge: the neutral glycans elute first, followed by the separation of acidic 2AB labeled
N
-glycans from monosialylated, disialylated, trisialylated, tetrasialylated and finally
pentasialylated species. Glycans of each charge state are further separated based on
their size and polarity. The retention time of each glycan charge state was confirmed
using 2AB labeled glycan standards (as shown in Figure 2). Separation of glycans is
based on charge, size, and polarity, which provides significant structural and quantitative
information. The chromatographic profiles shown in Figures 1
and 2, detected by
fluorescence detection, provide qualitative information about the separation of
N
-glycans
.
The structure of glycans present in each peak was determined from the LC-MS study
using the GlycanPac AXH-1 (1.9 µm) column as shown in the following section.
LC-MS Analysis of Native Glycans
The GlycanPac AXH-1 column is well su
and analysis of native glycans from MA
glycans not only eliminates the extra rea
during labeling, but also retains the origi
ambiguity imposed by the labeling reacti
native
N
-glycans from Bovine fetuin usin
native glycans were separated based on
ammonium formate/acetonitrile gradient
separation enables excellent MS and M
confirmation of the glycan structure of e
profiles are significantly different from th
especially higher sialic acid glycans. Ho
provide better and more MS/MS fragme
is useful for the analysis of both native a
amount of sample available. If the amou
analysis of unlabeled glycans using the
FIGURE 1. Separation of 2AB labeled
N
-glycans from Bovine fetuin by charge, size
and polarity.
FIGURE 2. Comparison of 2AB labeled
N
-glycans standards and 2AB-
N
-glycans
from fetuin.
LC-MS and LC-MS/MS Analysis of 2AB Labeled
N
-Glycan Using GlycanPac AXH-1
Column
The coupling of the GlycanPac AXH-1 column to MS was also explored. This is particularly
attractive as MS, with it’s ability to provide structural information, enables in
-depth analysis
of complex glycans. 2AB labeled
N
-glycans from bovine fetuin were separated on the
GlycanPac AXH-1 column and analyzed on a Q Exactive mass spectrometer. Data-
dependant MS/MS spectra were acquired on all precursor ions (z< 2) and SimGlycan
software was used for glycan structural elucidation. A representative example of the
analysis is shown in Figure 3
.
The detailed structural information obtained from the MS/MS
data further validated the ability of the GlycanPac AXH-1 column to separate glycans
based on charge, size, and polarity. However, coelution of different charge state glycans
(Figure 4) is common with other commercially available HILIC columns.
FIGURE 4. LC-MS analysis of 2AB lab
commercial amide HILIC column (1.7
FIGURE 3. LC-MS analysis of 2AB lab
GlycanPac AXH-1 (1.9 µm) column wi
Prozyme is a registered trademark of ProZyme, Inc.
International. All other trademarks are the property of
This information is not intended to encourage use of
property rights of others.


