
Determination of Digoxin in Serum by Liquid
Chromatography–Tandem Mass Spectrometry
François-Ludovic Sauvage
1
, Pierre Marquet
1,2
1
Department of Pharmacology-Toxicology, University Hospital, Limoges, France.
2
Laboratory of Pharmacology, Faculty of Medicine, University of Limoges, France.
Application
Note: 384
Key Words
• TSQ Quantum
• Clinical
Research
• Toxicology
Introduction
Digoxin is a cardiac glycoside that can be used at very low
concentrations. Identification and quantitation of this
compound necessitate a sensitive and specific method.
This study aims to describe a method using liquid chro-
matography/ tandem mass spectrometry and permitting to
quantify digoxin at low concentrations for research appli-
cations.
Goal
The goal of this study was to identify and quantify
digoxin in serum. This report demonstrates the use of the
TSQ Quantum for this application.
Experimental Conditions/Methods
Chemicals and Reagents
Digoxin and 3-aminophenylsulfone (internal standard)
were purchased from Sigma. Ammonium formate and
formic acid (>99 % pure) were also purchased from
Sigma. All reagents and solvents used in the extraction
procedures were of analytical grade.
Sample preparation
To 1 mL of serum were added 50 µL of a 2.5 µg/mL
aqueous solution of 3-aminophenylsulfone (Internal
Standard), 1 mL of a solution of pH 9.50 carbonate
buffer and 8 mL of Ether-Dichloromethane-Isopropanol
(30:40:30 by volume). The tubes were vortex-mixed and
shaken on an oscillatory mixer. After centrifugation at
3,400 g for 5 min, the organic phase was poured in a
conical glass tube and evaporated under a stream of
nitrogen at 37°C. The dried extracts were reconstituted
in 50 µL of acetonitrile : pH 3.0, 2 mmol/L ammonium
formate (30:70 by volume) and 10 µL were injected into
the chromatographic system.
Instrumentation Methods
HPLC Conditions
The chromatographic system consisted of a CTC HTS
PAL Autosampler kept at 6°C and a binary high-pressure
pump. A C18, 5 µm (50 2.1 mm) column, maintained at
25°C, was used with a linear gradient of mobile phase A
(pH 3.0, 2 mmol/L ammonium formate) and mobile phase
B (ac
etonitrile:pH3.0, 2 mmol/L ammonium formate
(90:10; v/v)), flow rate of 200 µL/min, programmed as
follows: 0-1.2 min, 20% B; 1.2–8.2 min, 20 to 80% B;
8.2–10.2 min, 80% B; 10.2–10.7 min, decrease from 80
to 20% B; 10.7–13 min, equilibration with 20% B.
MS Conditions
Mass Spectrometer: Thermo Scientific TSQ Quantum
Source: ESI mode
Ion Polarity: Positive
Spray Voltage: 3800 V
Sheath/Auxiliary gas: Nitrogen
Sheath gas pressure: 30 (arbitrary units)
Auxiliary gas pressure: 30 (arbitrary units)
Ion transfer tube temperature: 250°C
Scan type: SRM
Collision gas: Argon
Collision gas pressure: 1.5 mTorr
SRM Conditions
Settings were optimized by infusing at 5 µL/min a 1 µg/L
solution containing the studied compound in acetonitrile:
pH 3.0, 2 mmol/L ammonium formate (30:70, by
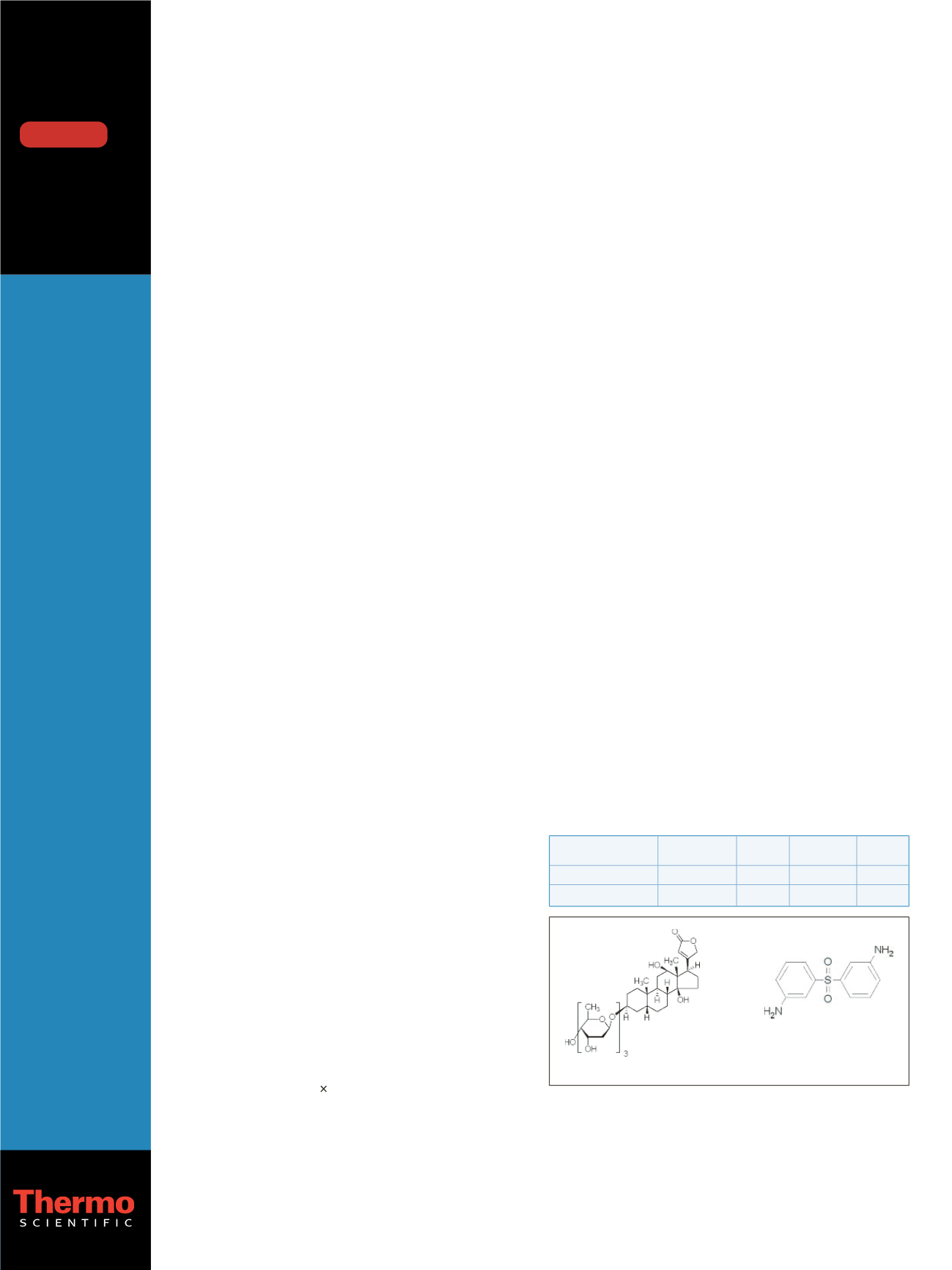
volume). The structure of these compounds is shown
in Figure 1.
Quantification Collision Confirmation Tube lens
Compounds
transition
energy transition voltage
Digoxin
798.5/651.4
20 798.5/781.5 84
3-aminophenylsulfone 249.1/93.2
24
126
Digoxin
3-aminophenylsulfone
Figure 1: Structures of the investigated compounds
DOWNLOAD


















