34
Details of characterization test solutes and their physiochemical properties (pK
a
and log D values obtained
from
. Chromatographic conditions as follows: mobile phase 90:10 (v/v)
acetonitrile:ammonium acetate (20 mM on the column, pH 4.7); flow rate: 0.5 mL/min; UV detection: 254 nm;
Injection volume: 5 µL; column temperature: 30 °C
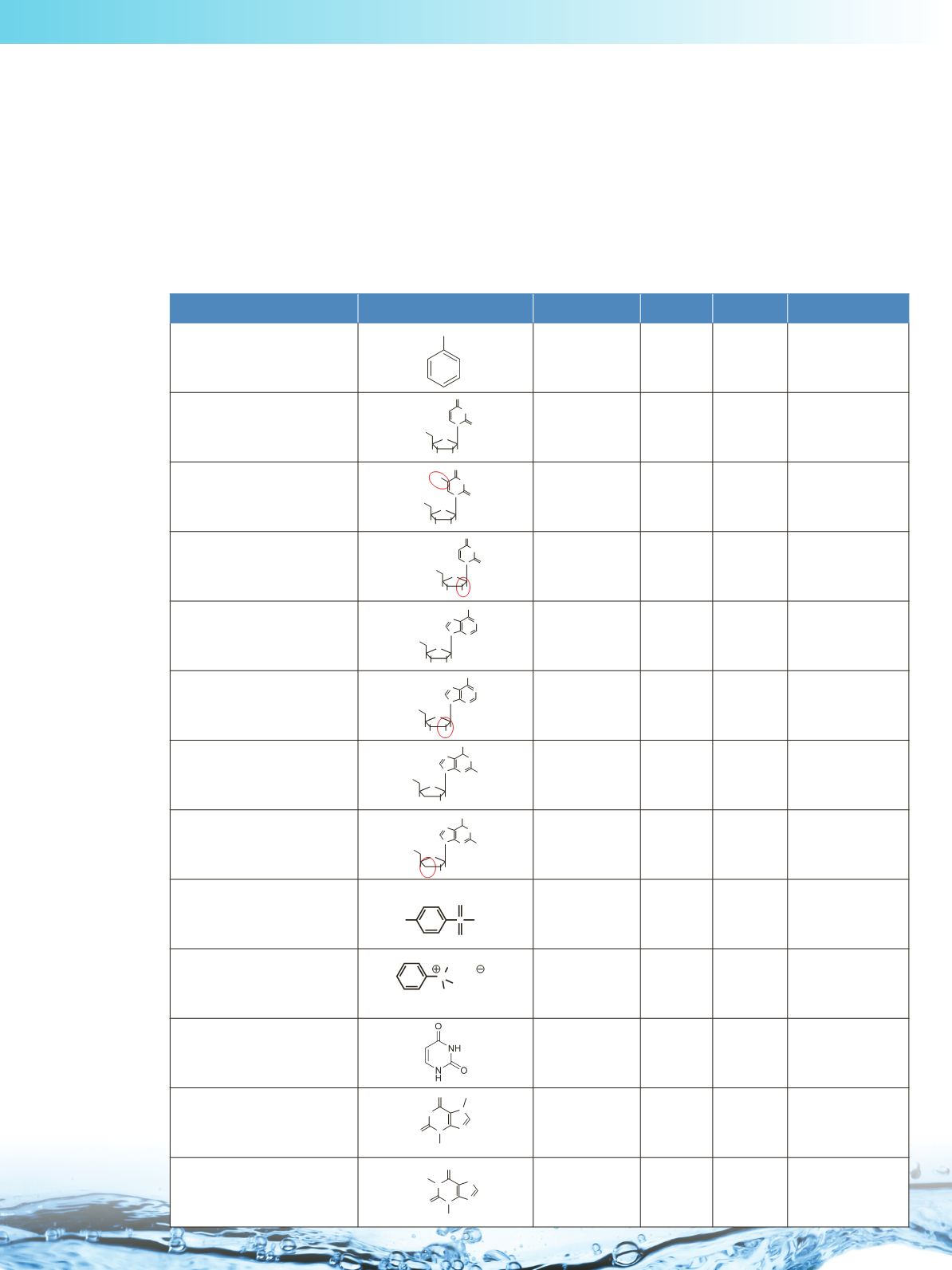
Stationary Phase Characterization Details
Chromatographic Probes
Molecular Structure
Variable
pK
a
Log D Separation Factors
Toulene
t
o
marker
na
2.72
all
Uridine
Hydrophobic/
hydrophilic
interaction
12.6
-1.58
α
(CH
2
),
α
(OH), k(U)
5-Methyluridine
Hydrophobic
interaction
12.0
-1.02
α
(CH
2
)
2'-Deoxyuridine
Hydrophilic
interaction
13.9
-1.26
α
(OH)
Adenosine
Configurational
isomers selectivity
13.9
-1.03
α
(V/A)
Vidarabine
Configurational
isomers selectivity
13.9
-1.02
α
(V/A)
2'-Deoxyguanosine
Positional isomers
selectivity
13.5
-1.14
α
(2dG/3dG)
3'-Deoxyguanosine
Positional isomers
selectivity
13.5
-1.14
α
(2dG/3dG)
Sodium p-toluenesulfonate
Anion exchange
selectivity
na
0.88
α
(AX)
N,N,N-
trimethylphenylammonium
chloride
Cation exchange
selectivity
na
-2.31
α
(CX)
Uracil
Hydrophilic
interaction
13.8
-1.08
α
(AX),
α
(CX)
Theobromine
Acidic-basic
nature of
stationary phase
10
-1.06
α
(Tb/Tp)
Theophylline
Acidic-basic
nature of
stationary phase
8.6
-0.5
α
(Tb/Tp)
O
O
OH
HO
H
H H
H
O
NH
N
OH
O
O
OH
HO
H
H H
H
O
NH
N
OH
H
3
C
O
O
OH
HO
H
H H
H
O
NH
N
H
N
O
OH
HO
H
H
H
N
N
N
OH
H
NH
2
N
O
OH
HO
H
H
H
N
N
N
H
HO
NH
2
N
O
OH
HO
H
H
H
N N
H
H
O
NH
NH
2
N
O
H
HO
H
H
H
N N
H
O
NH
NH
2
OH
H
3
C
ONa
O
O
N Cl
CH
3
CH
3
CH
3
S
H
3
C
ONa
O
O
N Cl
CH
3
CH
3
CH
3
S
HN
N
N N
O
O
CH
3
CH
3
H
N
N
N
N
O
O
CH
3
H
3
C
CH
3


