Africa-Other
+27 11 570 1840
Australia
+61 3 9757 4300
Austria
+43 1 333 50 34 0
Belgium
+32 53 73 42 41
Canada
+1 800 530 8447
China
+86 10 8419 3588
Denmark
+45 70 23 62 60
Europe-Other
+43 1 333 50 34 0
Finland/Norway/Sweden
+46 8 556 468 00
France
+33 1 60 92 48 00
Germany
+49 6103 408 1014
India
+91 22 6742 9434
Italy
+39 02 950 591
Japan
+81 45 453 9100
Latin America
+1 561 688 8700
Middle East
+43 1 333 50 34 0
Netherlands
+31 76 579 55 55
New Zealand
+64 9 980 6700
Russia/CIS
+43 1 333 50 34 0
South Africa
+27 11 570 1840
Spain
+34 914 845 965
Switzerland
+41 61 716 77 00
UK
+44 1442 233555
USA
+1 800 532 4752
©2012 Thermo Fisher Scientific Inc. All rights reserved. ISO is a trademark of the International Standards Organization.
All other trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. This information is presented as an
example of the capabilities of Thermo Fisher Scientific Inc. products. It is not intended to encourage use of these products in any
manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change.
Not all products are available in all countries. Please consult your local sales representative for details.
Application Brief 52300
Thermo Fisher
Scientific,Austin,TX
USA is ISO Certified.
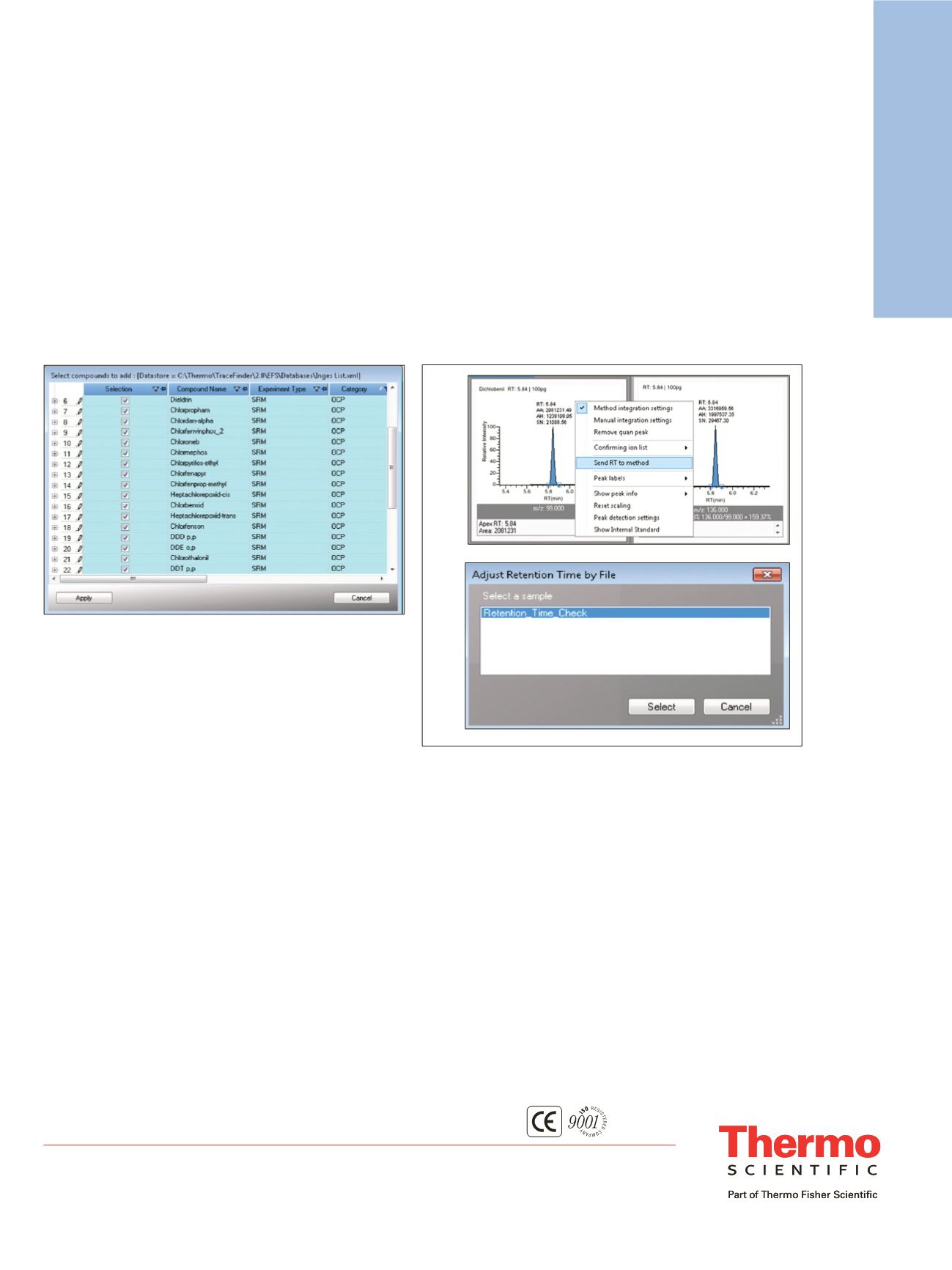
Automatic Retention Time Update
Since you now have a retention time-based instrument
method to acquire your transitions, and a TraceFinder
software method to process the data, how do you
accommodate the clipping of your column and all your
retention times change? If you sync the TraceFinder
method with the acquisition method, all you need to do is
run a standard and update your retention times in Data
Review (Figure 4). Most of these updated retention times
will be determined automatically through automatic peak
detection. The next time you run the method, the
acquisition list will be updated with the new retention
times, eliminating most of the manual work previously
needed to maintain a complex SRM instrument method.
For these complex methods, this can lead to a major time
savings in your daily work.
Compound-Based Scanning
Having the ability to sync the instrument method and the
processing method also allows for easy creation of subsets
of acquisition lists from the CDS. For instance, if you have
a large multi-residue method available, but you are only
interested in the organo-chlorine pesticides instead of the
full set for a particular analysis, simply select the category
for organo-chlorines when creating a processing method
from the CDS. This will not only create a processing
method with your selected compounds, but it will also
create the corresponding acquisition list, limiting the list
to just those compounds. This limited transition list will
increase the dwell time of the selected transitions, and
thus further increase the sensitivity of the TSQ 8000
GC-MS/MS system.
Figure 3. SRM, SIM and full scan rates based on peak width and
scans across the peak
A
B
Figure 4. With Method Sync, when you adjust retention times through
automatic processing in Data Review, retention times will also be
updated in your timed acquisition method. You can update the
retention times one at a time while you QC your data in Data Review
(a), or adjust retention times en masse from an acquired sample (b).
AB52300_E 06/12S


