6
Highly Sensitive, Robust MS-Based Workflow for Therapeutic Monoclonal Antibody Analysis from Complex Matrices
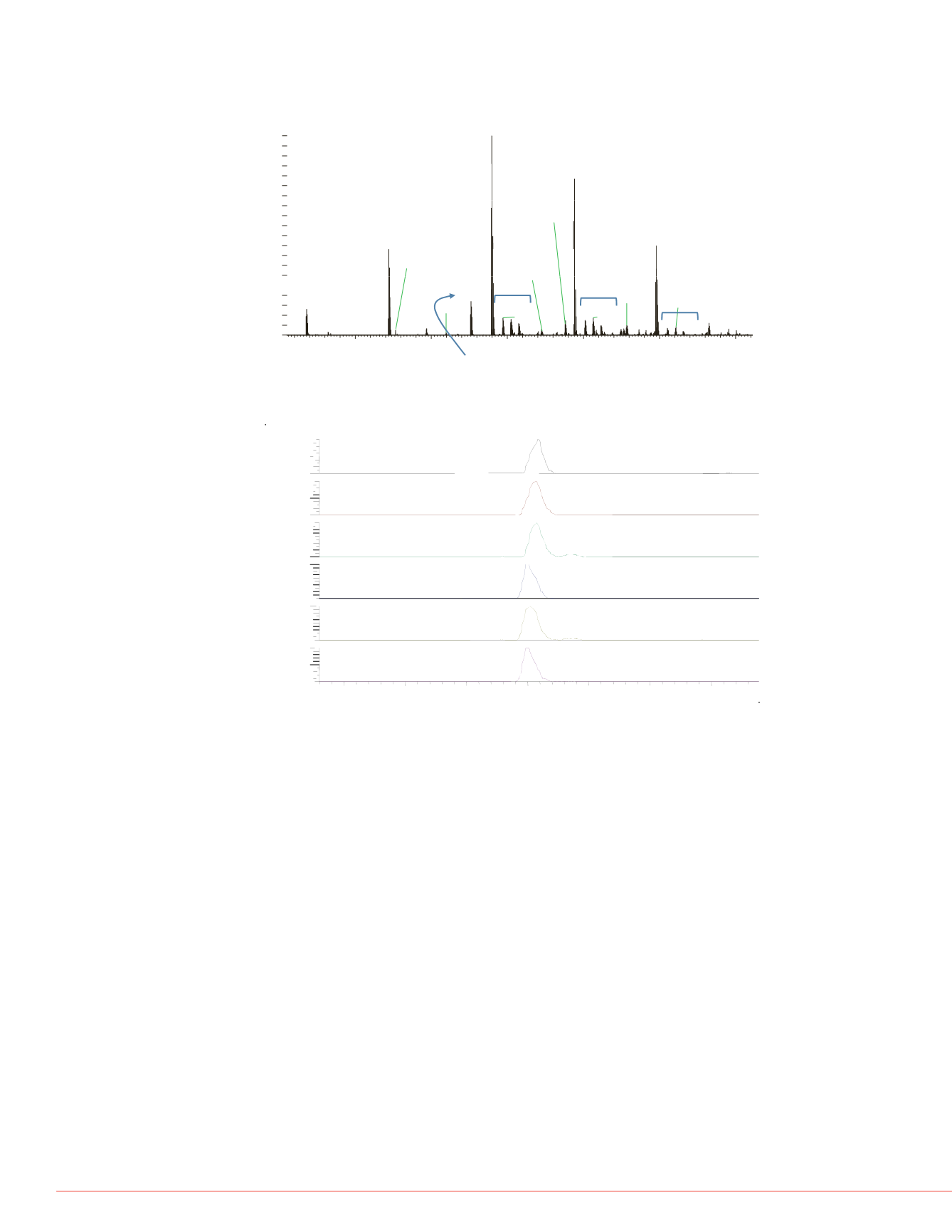
limumab from plasma. A) Base peak
s for three adalimumab peptides. E)
rd peptide at 200 fmol.
682.7032
NL: 1 06E8
FIGURE 5. Full MS trace showing the the adalimumab glycoform variation on heavy chain
peptide TKPREEQYNSTYR at 5 ng/mL. Note the ion clusters in brackets are salt adducts.
Hex3HexNAc4dHex
.
NL: 9.65E6
70
75
80
85
90
95
100
1039.7880
z=3
1093.8057
z=3
Hex4HexNAc4dHex
599.9702
NFYPR
,
+3
m
NL: 7.29E6
30
35
40
45
50
55
60
65
RelativeAbundance
1147.8234
z=3
972.0947
z=3
1087.9570
z=4
976.4959
z=1
1072.7785
3
Hex5HexNAc4dHex
Hex3HexNAc3dHex
TLPPSRDELTK
,
+4
.2 ppm
NL: 1.34E6
NL: 7.07E5
950
1000
1050
1100
1150
1200
m/z
0
5
10
15
20
25
1026.1132
z=3
918.0775
z=3
1106.4558
z=3
1047.1155
z=3
1182.4904
z=3
1128.4730
z=3
1160.8089
z=3
996.9253
z=4
z=
932.1917
z=4
1009.7373
z=3
Hex2HexNAc3dHex
30
35
40
umab HC at 5 ng/mL. Note the
S
Hex4HexNAc3dHex
FIGURE 6. Relative retention times and abundances of the observed glycoforms of the
HC peptide TKPREEQYNSTYR.
eavily glycosylated based on M
ation, D = deamidation.
Hex3HexNAc3dHex
Hex2HexNAc3dHex
3.77E4
1.27E5
ance
50
100
0
50
100
918.0762
972.0945
H 3H NA 4dH
Hex4HexNAc3dHex
2.83E5
8.96E4
50
100
0
50
100
Relative Abund
0
1039.7880
1026.1147
Hex4HexNAc4dHex
ex ex c ex
3.38E5
2.61E5
100
0
50
100
0
1093.8062
mumab LC at 5 ng/mL.
idation
.
Hex5HexNAc4dHex
7.0
7.5
8.0
8.5
9.0
9.5
10.0
Time (min)
0
50
1147.8237
Conclusion
The data demonstrates the capabilities of MSIA Streptavidin Workflow as a
reproducible and robust method for analysis of therapeutic antibodies.
The analytical detection limit of 5 ng/mL for adalimumab, using high flow LC, from
human plasma samples was observed.
MSIA Streptavidin Workflows overcome cross reactivity issues that plague traditional
i F t
ti
t
Ab b d th d b i
th t
t ti
in tryptic peptide D
152
-K
214
nor the
me hydrophobicities and thus
gener c c arge ng cap ure
ase me o s y us ng e arge an gen as an
affinity ligand.
The described method provides highly specific characterization data. In the
adalimumab model, multiple glycoforms were detected. However, this sets the
foundation for other high value characterization methods such as Drug Antibody
des: Coverage for adalimumab
ere not performed.
re, with trypsinization only 81% of
ces are LC-MS/MS exceptional.
,
Ratio determination of Antibody Drug Conjugates.
The MSIA Streptavidin Workflow provides a remedy to chronic issues observed with
therapeutic neutralization events and protein complexation.
The described method sets the foundation for quantitative analysis for the
L 500 ng/mL 5000 ng/mL
67% 78%
simultaneous determination of quantity, character and functionality of the targeted
therapeutic molecule.
77% 75%
eavy chain and light chain sequences.
SEQUEST is a registered trademark of the University of Washington. All other trademarks are the property of Thermo
Fisher Scientific and its subsidiaries. This information is not intended to encourage use of these products in any
manners that might infringe the intellectual property rights of others.
PO64136-EN 0614S


