5
Thermo Scientific Poster Note
•
PN-64082-ASMS-EN-0614S
Conclusions
mAb monomer and
MS under non-den
mM ammonium for
The Exactive Plus
of mAb at
m/z
350-
mAbPac SEC-1 col
separates Fab, an
acetonitrile, 0.1% f
References
1.
Lin, S., Rao, S., Th
Monoclonal Antibo
Variant Analysis. Pr
January 23
–
25, 20
2.
Valliere-Douglass J
Mass Determinatio
and F at Interchain
was achieved on a short SEC
oth dimer aggregate and monomer
deconvoluted spectra of
d 297,105 u (Figure 2c),
mass148,393 u and 148,554 u
d mass and calculated mass
spectively. In addition, the dimer
hetero-dimer of monomers at
denaturing condition using
c SEC-1 4 x 300 mm column and
chromatogram of mAb, (b) mass
.
FIGURE 4: SEC-MS analysis o
using 20% acetonitrile, 0.1% f
injected onto a MAbPac SEC-1
µL/min.
(a) extracted ion chrom
deconvoluted spectrum of Fc, (d
of Fab.
Analysis of mAb fragments by denaturing SEC-MS
Comprehensive analysis of the mAb post translational modifications, such as
deamidation, C-terminal lysine truncation, N-terminal pyroglutamation, methionine
oxidation, and glycosylation, requires complete digestion of the mAbs and sequencing of
all the peptides. However, “peptide mapping” is time consuming. A simpler and faster
way to analyze the mAb variants and locate the modifications is to measure the mass of
heavy chain and light chain, or Fab and Fc fragments. Heavy chain and light chain are
generated by the reduction of mAb. Fab and Fac fragments are generated by papain
digestion. For example, the glycan modification is located in the Fc region of the heavy
chain, glycan variants can be detected in the heavy chain and Fc fragment mass
profiles, while light chain and Fab fragment mass profiles should only show a single
polypeptide chain.
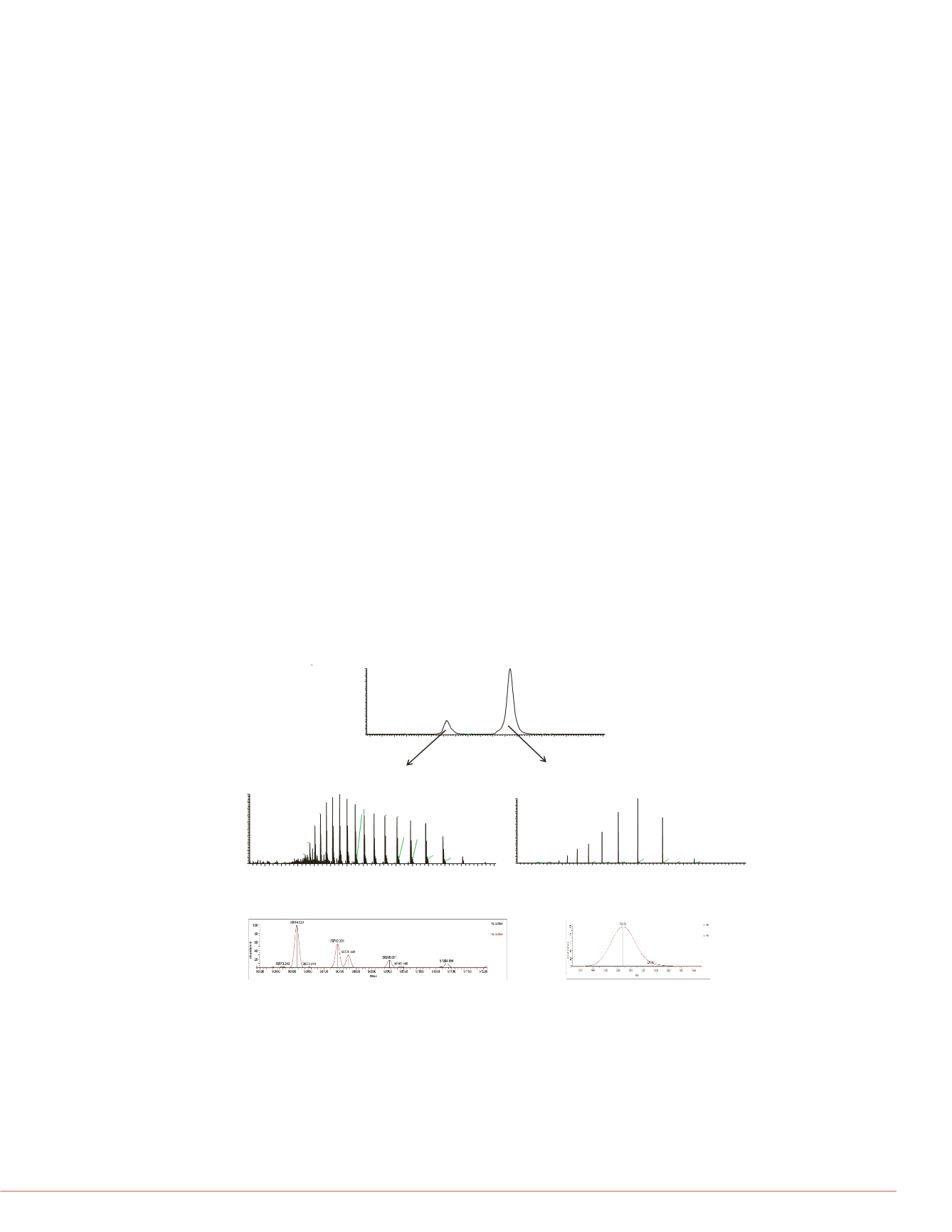
Figure 3 shows the SEC-MS analysis of HC and LC of mAb1 using 20% acetonitrile,
0.1% formic acid, and 0.05% TFA. Figure 3a shows the extracted ion chromatogram of
HC with
m/z
at 3163.70-3164.89 and LC with
m/z
at 2600.78-2601.88. Using this
denaturing eluent system, mAb HC elutes at about 10.15 min and mAb LC elutes at
about 12.71 min. Different mAbs have been tested and their HC and LC have similar
retention time. Therefore, denaturing SEC can be used as a platform method for the
separation of HC and LC of mAbs. Figure 3b shows the charge envelope of mAb HC in
the
m/z
range of 1900-3600 and Figure 3c shows the deconvoluted mass spectra of the
mAb HC, with a main peak at mass 50614.5 u and adjacent peaks at mass 50,742.3 u,
and 50,776.4 u, corresponding to a lysine variant and a different glycoform with 1
additional hexose. The lysine variant is located at the C-terminal of the HC. Figure 3d
shows the charge envelope of mAb LC in the
m/z
range of 1500-3500 and Figure 3e
shows the deconvoluted mass spectra of the mAb LC, with a single peak at mass
23,403.7 u. The mAb light chain is not glycosylated and does not have C-terminal lysine
variants. The intact mass of mAb is determined at mass148,029 u using the equation
2x(HC+LC)-8. The calculated mass is in good agreement with the measured mass at
mass 148,035 u.
Sigma is a registered trademark of Sigm
Scientific and its subsidiaries.
This information is not intended to encou
intellectual property rights of others.
FIGURE 3: SEC-MS analysis of mAb1 heavy chain and light chain under
denaturing condition using 20% acetonitrile, 0.1% formic acid and 0.05%
trifluoroacetic acid.
mAb was injected onto a MAbPac SEC-1 4 x 300 mm column
and the flow rate was set at 200
µL/min.
(a) extracted ion chromatogram of heavy
chain (HC) and light chain (LC), (b) mass spectrum of heavy chain (HC), (c)
deconvoluted spectrum of heavy chain (HC), (d) mass spectrum of light chain (LC),
(e) deconvoluted spectrum of light chain (LC).
Using the same chromatographi
off the SEC column at 9.94 and
good as the HC and LC due to t
size. Figure 4b shows the char
Figure 4c shows the deconvolut
52,752.9 u and adjacent peaks
lysine variant and a different gly
charge envelope of Fab in the
deconvoluted mass spectra of t
mass of mAb is determined at
calculated mass is more than 7
indicating an additional fragmen
14
16
18
20
13.99
14.04
.92
14.14
14.23
15.96
6800
7200
7600
68.60
7432.03 7616.74
7251.92
6768.23
(a)
(b)
3
(c)
1+Lys
G2
egate and monomer under non-
was injected onto a MAbPac
set at 50
µL/min.
(a) extracted ion
s spectrum of mAb dimer, (c)
trum of mAb monomer, (e)
er
)
+27
+26
+25
+24
+28
G1
G0
RT:
6.88 -16.56
7.0
7.5
8.0
8.5
9.0
9.5
10.0 10.5 11.0 11.5 12.0 12.5 13.0 13.5 14.0 14.5 15.0 15.5 16.0 16.5
Time (min)
0
10
20
30
40
50
60
70
80
90
100
RelativeAbundance
12.71
10.15
8.68
10.81
8.89
13.75
11.00
8.55
9.52
7.02
9.17
11.57
14.0114.31 15.03
7.41
8.11
7.84
1200
1400
1600
1800
2000
2200
2400
2600
2800
3000
3200
3400
3600
3800
4000
m/z
0
10
20
30
40
50
60
70
80
90
100
RelativeAbundance
2601.34
2341.31
2926.39
2128.55
1951.24
1801.26
1672.69
3344.41
1561.09
2619.36
2946.82
3901.71
3121.58
3601.60
2385.90
1463.66
2169.16
1988.65
3367.77
2754.36
1322.72
1101.60
1200
1400
1600
1800
2000
2200
2400
2600
2800
3000
3200
3400
3600
3800
4000
m/z
0
10
20
30
40
50
60
70
80
90
100
RelativeAbundance
2109.96
2201.64
1947.72
2301.67
2531.73
1875.65
2411.25
2664.91
2812.90
2978.34
3164.42
1808.68
3375.33
1746.42
1688.25
3616.42
1557.72
2323.12
3006.03
2839.03
3193.70
1110.59
1340.65
3406.59
3894.42
3345.21
Full MS of heavy chain (HC)
Full MS of light chain (LC)
(a)
(b)
(d)
G0
G1
G0+Lys
G1+Lys
G2
(c)
(e)
+10
+9
+8
+11
+12
+7
+13
+20
+18 +16
+14
0
1
2
3
4
0
10
20
30
40
50
60
70
80
90
100
RelativeAbundance
0.04 1.21 2.09 2.97 3.34 4.55
1000
1200
1400
1600
1800
2000
2200
2400
2600
2800
3000
m/z
0
10
20
30
40
50
60
70
80
90
100
RelativeAbundance
1885.02 2029.96
2198.99
1820.08
2294.58
1759.45
2398.80
2513.00
1702.73
2638.61 2777.40
2931.65
31
1649.52
1541.93
2958.26
1501.57
1167.42
1064.47
Full MS of Fc
(a)
(b)
(c)
G0
G0+Lys
+20
+18


