4
Highly Sensitive, Robust MS-Based Workflow for Therapeutic Monoclonal Antibody Analysis from Complex Matrices
FIGURE 2. LC-MS chromato
chromatogram. B-D) Extract
Extracted ion chromatogra
100
ith a solution of biotinylated TNF-α to
umn surface Plasma samples spiked
Intact Analysis of Reduced Adalimumab
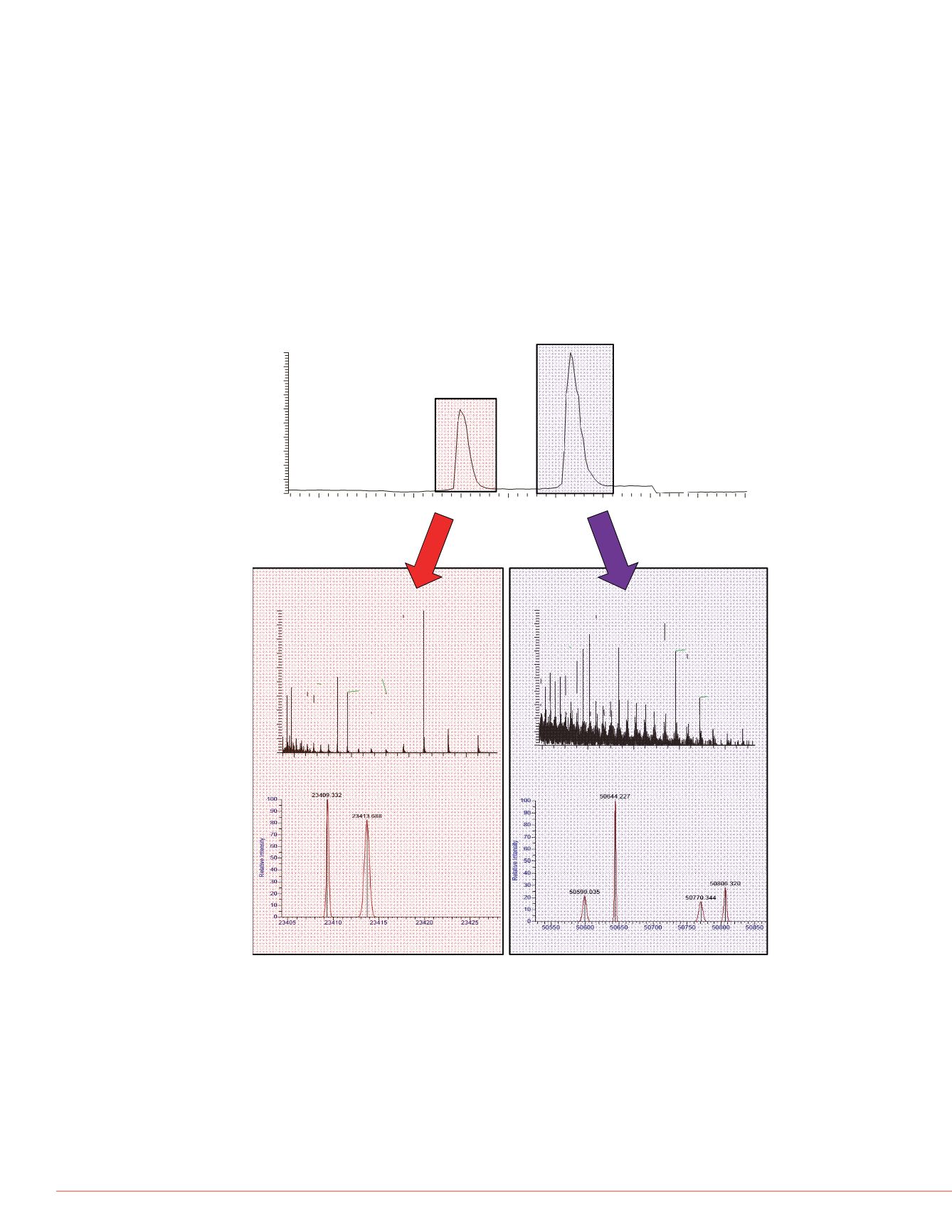
Figure 1
shows the elution profile of adalimumab (500 ng spiked into plasma at 1
µg/mL) purified from human plasma. The Ab was reduced to heavy chain (HC) and
60
80
100
0
20
40
60
80
NQV
A
B
.
F-α-derivatized MSIA Streptavidin
dalimumab was achieved by repetitive
ample solution though the functionalized
ification, the MSIA Streptavidin
h d d th t
t d ith l ti
light chain (LC). Deconvolution of the LC mass spectrum generated provided two
masses, 23409.33 and 23413.68. This 4 Da separation is indicative of incomplete
reduction of the two disulfide linkages of the LC. The mass at 23413.72 is within 1 Da
of the theoretical LC average mass of adalimumab. Deconvolution of the HC mass
spectrum gave a mass at 50644 22 which is suggestive of a modification by loss of the
FIGURE 1: Intact analysis of reduced adalimumab A) Base peak chromatogram of
reduced adalimumab showing the elution profiles of light chain (LC) and heavy chain
0
20
40
60
80
100
RelativeAbundance
0
20
40
C
re was e an en rea e w e u on
y purification steps were automated by
tomated liquid handler.
b underwent two types of treatment just
dalimumab either subjected to (1)
. ,
C-terminal lysine and the addition of one N-linked glycan. The mass at 50806.32
represents the addition of a hexose group.
HC
(HC). B) and C) Raw MS spectra for LC and HC, respectively. D) The deconvolved
average mass (M+H) of LC. E) The deconvolved average mass (M+H) of HC.
80
100
0
20
40
60
80
100
D
E
stion (120 ng of trypsin) for bottom up
calibrated through the addition of 5.95
tion (PRTC) peptide mixture to each
60
70
80
90
100
undance
8.66
7.49
NL: 8.77E7
LC
A
5
10
15
0
20
40
60
FIGURE 3. Bottom-up seque
imumab (ranging from 5-500 ng/mL in
0
10
20
30
40
50
RelativeAb
sequence underlined in red
full scan data. C = carbamid
urification (described above) and then
ptide fragments were injected onto a
00 mm column heated to 70 °C.
radient of 2-35% formic acid (0.1%) in
0 RSLC.
6.0
6.5
7.0
7.5
8.0
8.5
9.0
9.5
10.0
10.
Time (min)
b, 1.8
μ
g/mL was recovered from
™ ProSwift™ column (RP-4H 0.5 mm x
nd light chain were eluted at 200 µL/min
) in acetonitrile in 8.2 minutes on an
80
90
100
2129.2008
80
90
100
1447.9270
1236.2184
1535.6830
1126 3682
1583 5670
B
C
ass spectrometer.
10 d t d d t
th d F ll
30
40
50
60
70
Relative Abundance
1378.0564
1233.0984
1464.2771
1801.7598
2342.0249
20
30
40
50
60
70
RelativeAbundance
.
.
1689.2232
1809.6181
FIGURE 4. Bottom-up seque
C = carbamidomethylation,
op a a- epen en me o . u
70,000 (FWHM) at
m/z
200 and an AGC
ired at a resolving power of 17,500
of 1E5 with a normalized collision
sion duration.
1000
1500
2000
2500
m/z
0
10
20
2602.2274
1000
1200
1400
1600
1800
m/z
0
10
D
E
zed with a full scan taken from m/z 900-
) at
m/z
200 and an AGC target value of
Note that we did not expect
light chain tryptic peptide F
6
ST® HT in Thermo Scientific
TM
against a database of adalimumab
15 common contaminant proteins.
rsor tolerance and a 0.02 Da fragment
TABLE 1. Bottom-up analysi
from varying concentration
incompatibilities with C18 c
the heavy chain and 85% of
of N-termini, oxidation of methionines,
idation of asparagines and glutamines.
lator.
Thermo Scientific
TM
Protein
pect™ algorithm
Bottom-Up Analysis of Adalimumab
Plasma samples were spiked with adalimumab at 5 ng/mL, 10 ng/mL, 50 ng/mL,
500 ng/mL and 5000 ng/mL. Adalimumab was retrieved from matrix by using TNF-
5 ng/
HC % Sequence
Coverage
79%
.
aditional ligand binding with MS
α-derivatized MSIA Streptavidin D.A.R.T.’S. Then, the purified analyte was subject
to reduction, alkylation, and trypsin digestion prior to the LC-MS/MS sequence
coverage determination and PTM analyses.
LC % Sequence
Coverage
85%
*
The percentage sequence cove
t and reproducible method to therapeutic
the therapeutic mAb for its target
as a part the general analysis, while
lytical flexibility over other developing


