
6
Improving Label-Free Quanti cation of Plasma and Serum Proteins Using a High-Resolution Hybrid Orbitrap Mass Spectrometer
Conclusion
The development of the real-time state modeled data acquisition provides
quantification of peptide species at lower concentrations and lower signal
thresholds (below 1 e
4
).
Real-time state modeled data acquisition results in at least 50% more
identifications than MS1 quantification below 10 fmol.
This novel data acquisition scheme provides higher sensitivity and selectivity of
peptides in a label-free complex matrix – ideal for a biomarker discovery workflow.
References
1. Prakash, A.; Peterman, S.; Frewen, B.; Kuehn, A.; Ciccimaro, G.; Schroeder, T.;
Vasicek, L.; Hood, B.; Bomgarden, R.; Krastins, B.; Sarracino, D.; Byram, G.;
Vogelsang, M.; Worboys, J.; Jorgensen, C.; Conrads, T.; Lopez, M. Improving
throughput for highly multiplexed targeted quantification methods using novel API-
remote instrument control and state-model data acquisition schemes.
61
st
ASMS Conference on Mass Spectrometry and Allied Topics, Minneapolis,
MN, June 9–13, 2013. Poster TP08 – Peptides: Quantitative Analysis I, poster
number: 131, Tuesday, Halls B&C.
All trademarks are the property of Thermo Fisher Scientific and its subsidiaries.
This information is not intended to encourage use of these products in any manners that might infringe the
intellectual property rights of others.
t each femtomolar level, for
cation (B, D, F). (A) MS1 peak
profile view of VGDANPALQK.
fmol level. (C) and (D) MS1 and
GFQNALIVR. Insets (C) and
and (F) MS1 and MS2 peak
et (E) shows an expanded view
ring peak. Inset (F) shows MS2
(H) MS1 and MS2 peak profiles,
VLGPVR. Inset (H) shows an
s for the representative
MS2
10 fmol
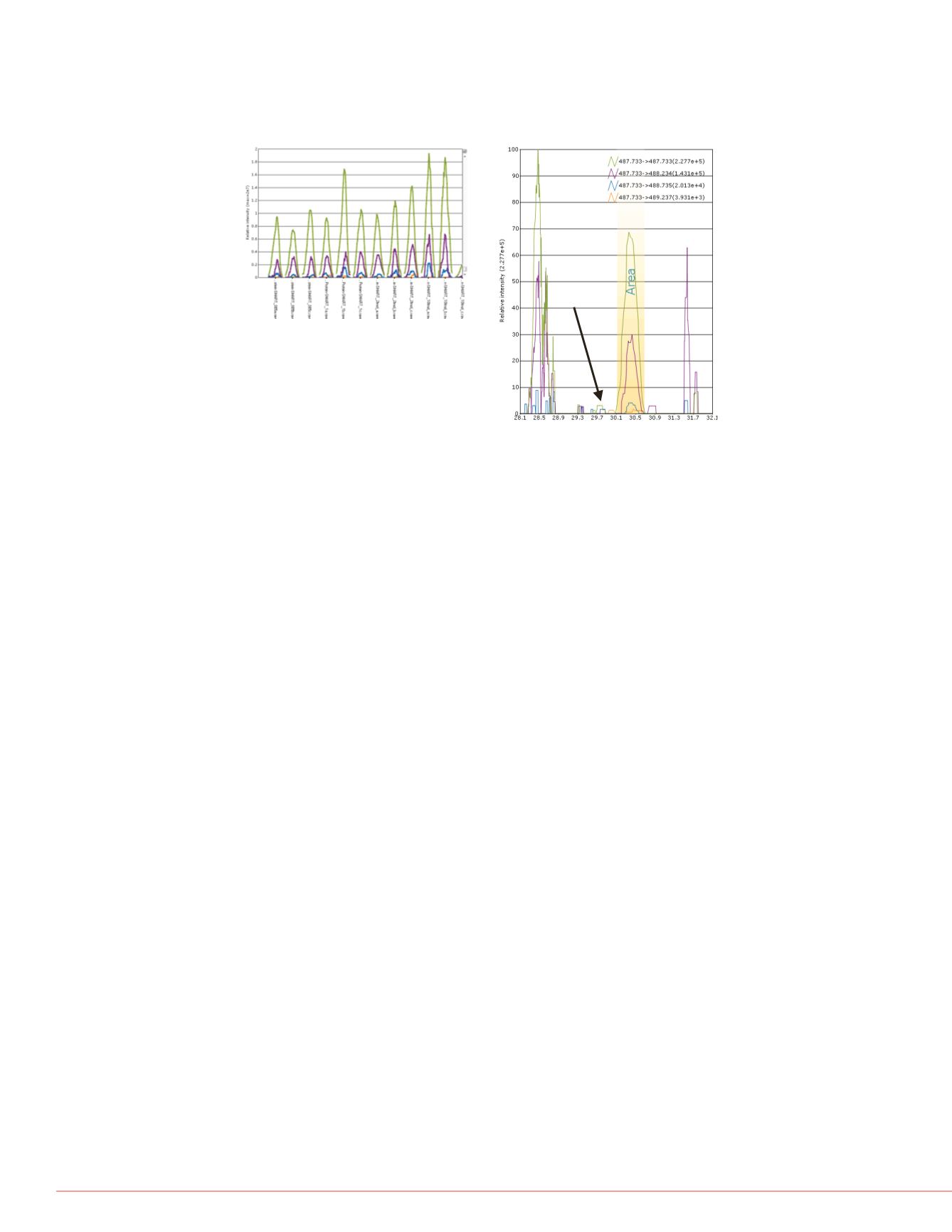
FIGURE 5. (A) False positive MS1 peak profiles for peptide DLGEEHFK at 0.5 to
10 fmol level. (B) XIC for 487.733 isotopes, illustrating probable isobaric
contaminants; (arrow) points to observed elution time for peptide DLGEEHFK at
higher levels.
A
B



















