
3
Thermo Scienti c Poster Note
•
PN ASMS13_W457_DSarracino_E 07/13S
ins for sepsis in an
in vitro
toxic ligands secreted by
alkylated, digested with trypsin.
atography-tandem mass
entified by Thermo Scientific™
Thermo Scientific™ Pinpoint™
levant kinase and pathway
roteins.
polysaccharides (LPS), are
protein profiling of mononuclear
the protein dynamic range
e responsive to many immune
er discovery experiments.
directly in tubes used for the
r changes in either secreted
n changes in the PBMC cell
li
, we chose the corresponding
cific Toll like receptor 4(TLR4) in
innate immune response
ts that are either proteolytic
e large number of human
ations (PTM) represent a
ffect, the simplest sample
ble results.
ted into BD Vacutainer™ CPT
e with IRB approval. Buffers
lood collection tubes using a
00 µL of phosphate buffered
lated tubes. LPS-EB Toll Like
ded to a concentration of
le blood.
Results
In order to allow the detection of diff
instrumentation should provide eno
simultaneously providing MS/MS fra
peptides as possible. In this experi
confidence containing >250 phosph
oxidations (other than methionine) t
Pinpoint software has the advantag
library import. In this example, all ki
analysis. Once selected, proteins th
verified using Pinpoint software (Fig
specific proteins of interest (Figure
(Figure9) MAPKKK 15, can be sele
After incubation at 37 ºC for 30 min the cells were isolated according to the
manufacturerʼs instructions for a total exposure time of 60 min. Rinsed cell pellets
(~2 mg) were denatured in 350 µL of 8M Urea 300 mM Tris-HCl 2.5% n-propanol
10 mM Dithiothreitol, reduced/alkylated, diluted to 2 mL with 50 mM Tris, 5 mM CaCl2
and digested overnight with 20 µg of Pierce Trypsin Protease, MS Grade.
Liquid Chromatography
Peptide retention time standards were added and the samples were loaded into 96-well
plates onto a Thermo Scientific™ EASY-nLC™ 1000. Separations were done on a
Thermo Scientific™ Dionex™ PS-DVB trap column, (5 µm particle, 300Å pore, 150 µm
x 12 cm) connected in a “vented t” configuration to a 5 µm particle, 200Å pore C18AQ
100 µm x 50 cm packed tip resolving column in a Thermo Scientific™ Nanospray
Flex™ Ion Source on a hybrid Thermo Scientific™ Orbitrap Velos Pro™ MS. Stepped
Flow and gradient from 4-50%B at 650 nL/min over 205 min. Buffer A is 2% Methanol
0.2% formic acid, water(v/v). Buffer B is 10% water, 10% isopropanol, 80% acetonitrile
0.2% formic acid (v/v), all solvents are Thermo Scientific™ Optima™ LC-MS grade.
Portions of each of the TLR4 digests (Low and High stim) pooled for library creation
were fractionated into 12 fractions of 1.8 ml, on a 4.6 mm x 25 cm PS-DVB column
8 µm particle 300A pore, buffer A: 100 mM ammonium formate, 58 mM ammonium
hydroxide, Buffer B: 29 mM ammonium hydroxide in 91% acetonitrile 9% water (v/v),
using a flow rate of 1 mL/min in a gradient to 45% B (Figure 2).
Mass Spectrometry
For mass spectrometric analysis, a data-dependent top 25 method has been used .
Full MS scans acquired at a resolution of 100,000 using a 1e6 target value, with
dependent scans analyzed in the linear ion trap with normal scan resolution.
Uncharacterized charge states and + 1 charge states are rejected. Chromatography
phase triggering with monoisotopic fitting was used with a peak width of 40 s and a
minimum peak threshold of 3.5e4. The maximum inject time allowed for MS/MS scans
was set to 100 ms. Dynamic exclusion is turned on using a peak width of 60 s.
Data Analysis
Full-scan comparisons were made using Pinpoint software, and MS/MS spectra were
processed by Proteome Discoverer software using The Mascot® search engine. Two
different peptide identification strategies were used. The simple search method (Figure
3) only searches for high-confidence, tryptic peptides and phosphopeptides. The more
complex search strategy (Figure 4), breaks the PTM search strategy into multiple
nodes, where small groups of PTMs, likely to occur on the same peptide, are searched
in each node. This allows for higher-confidence assignments due to the reduced size
of each database, albeit at an increased search computational time. Pathway
information was processed using Thermo Scientific™ ProteinCenter™ software (not
shown). Pinpoint software allows for the import of spectral libraries which can be
obtained from data from both unfractionated and fractionated samples provided the
chromatography in all samples is reproducible and retention times are consistent.
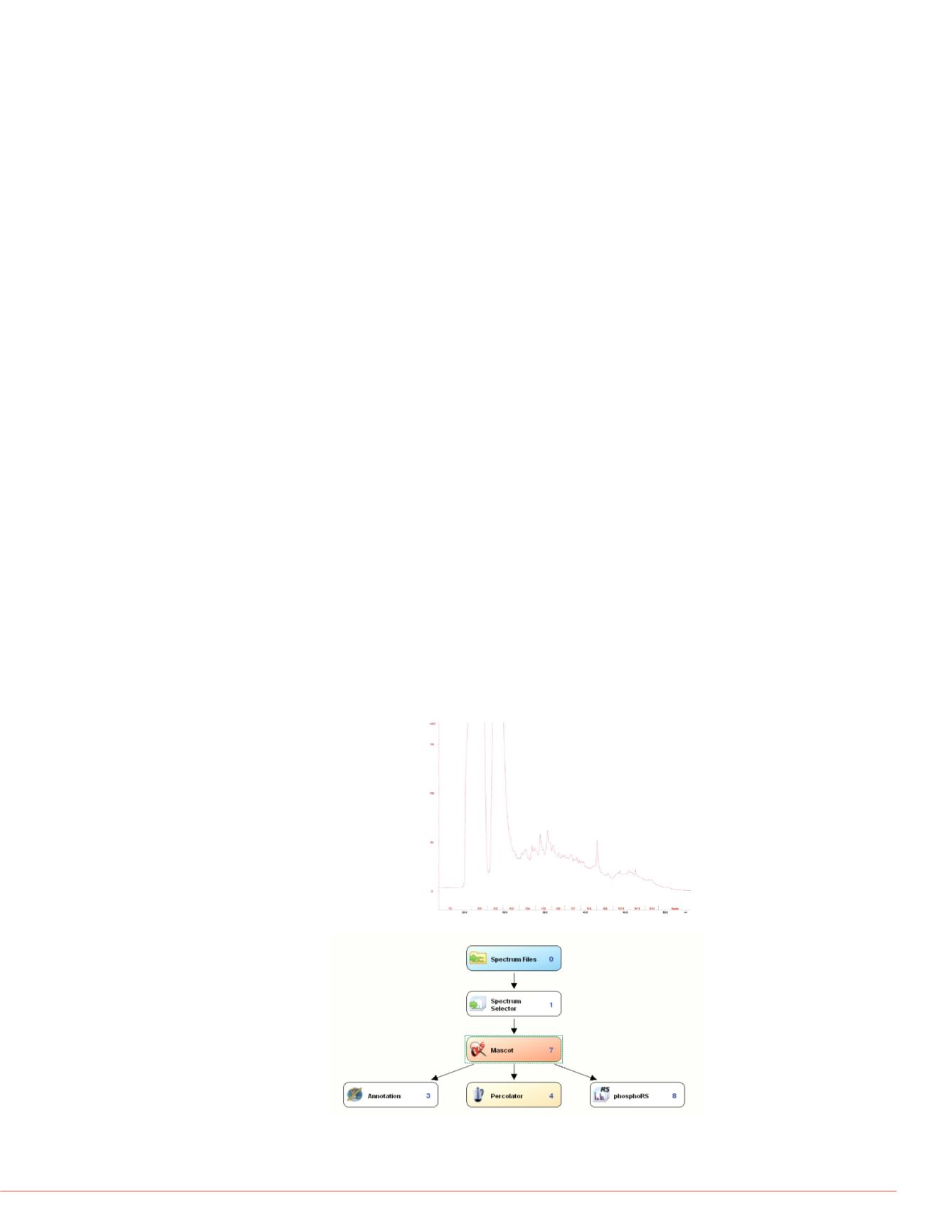
FIGURE 4. Search workflow for m
into groups of most likely to occ
computationally intensive and w
analyzed MS2. Many modification
deamidations, semi-tryptic, and d
other search engines without co
FIGURE 5. Results from different
then be brought into Pinpoint sof
FIGURE 2. High pH reverse
phase fractionation for library
peptide fractionation
FIGURE 3. Search
workflow for protein
phosphorylation.



















