
5
Thermo Scientific Poster Note
•
PN-64139-ASMS-EN-0614S
n sites for EGFR peptides.
osphorylation sites for EGFR
)PSGEAPNQALLR peptide
ing Thermo Scientific Pierce
from two cell lines with
din coated magnetic beads,
LC-MS/MS to assess sequence
HEK293
ve
l
Anti-target
Ab
Negative
Control
16%
0%
68%
6%
82%
0%
62%
0%
36%
0%
0%
0%
693-
Phosphothreonine
by PKD/PRKD1
phoserine
y₁₄⁺-P
1448.85
b₁₇⁺
1826.83
y₁₆⁺-H₂O
1754.86
b₁₈⁺-P
1842.06
y₁₅⁺-P
1545.87
b₁₈⁺
1940.12
y₁₃⁺-H₂O
1415.81
₁₂⁺
3.60
y₁₃⁺
1433.92
y₁₅⁺
1643.90
1400
1600
1800
2000
RT: 31.37
QALLR
FIGURE 5. Detection and quantitation limits of EGFR, AKT2, AKT1, PTEN, PIK3CA
and PIK3R1 peptides.
All six targets were monitored with linear quantification. EGFR, AKT2, PTEN, PIK3CA
and PIK3R1 peptides were quantified from 3.9 fmol to 1000 fmol.
Target
Peptide No.
LOD (fmol) LLOQ (fmol) ULOQ (fmol) Linearity (R
2
)
EGFR
Peptide 1
0.2
3.9
1000
0.9977
Peptide 2
0.2
3.9
1000
0.9997
AKT2
Peptide 1
0.2
3.9
1000
0.9998
Peptide 2
3.9
15.6
1000
0.9599
AKT1
Peptide 1
3.9
15.6
1000
0.9541
PTEN
Peptide 1
0.2
3.9
1000
0.9999
Peptide 2
0.2
3.9
1000
0.9997
PIK3R1
Peptide 1
0.2
3.9
1000
0.9997
Peptide 2
0.2
3.9
1000
0.9999
PIK3CA
Peptide 1
0.2
3.9
1000
0.9981
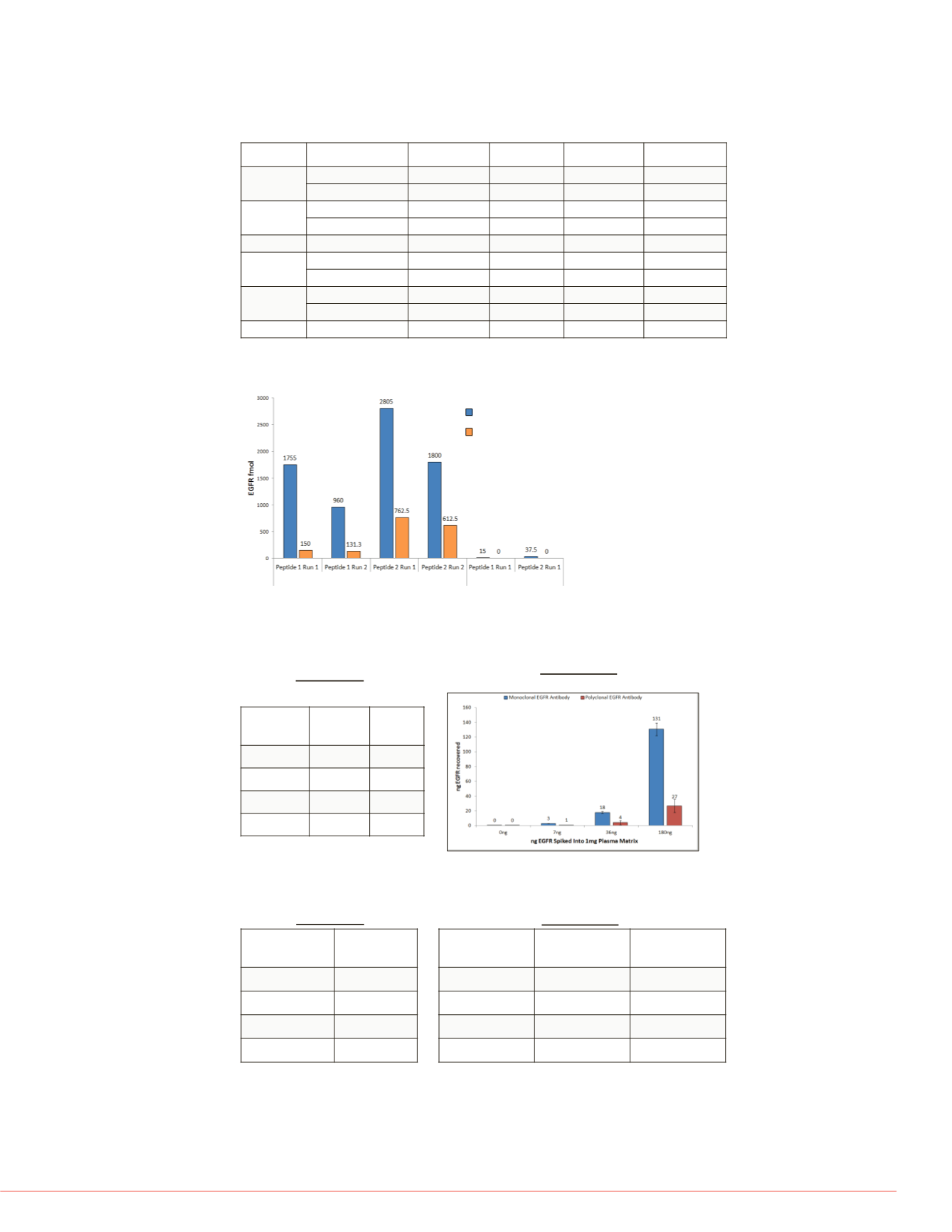
FIGURE 6. Quantitation of EGFR peptides by targeted MS.
Enrichment of EGFR from two cell lysate allowed for quantitation of two unique EGFR
peptides by targeted MS. Better recovery was observed with Pierce Streptavidin (SA)
Magnetic beads compared to Dynabeads MyOne Streptavidin T1 beads.
Immunoprecipitation to targeted MS research application (nLC-SRM/MS)
After enrichment by IP, SRM assays enabled the quantitation of EGFR, AKT2, AKT1,
PTEN proteins in the low fmol range.
FIGURE 7. Recovery of recombinant EGFR (rEGFR) from plasma matrix.
rEGFR spiked into 1mg plasma is detected and quantitated at >7ng (52 fmol).
Monoclonal antibody recovered more rEGFR.
rEGFR
Mono
Ab
Poly
Ab
0 ng
0%
0%
7 ng
3%
2%
36 ng
20%
12%
180 ng
42%
26%
nLC-MS/MS
(EGFR % Sequence Coverage)
nLC-SRM/MS
(EGFR Peptide Quantitation)
Pierce™ Streptavidin Magnetic Beads
Dynabeads
®
MyOne™ Streptavidin T1
A431
HEK293
Conclusion
Immunoprecipitation usin
resulted in a higher yield
using directly immobilized
Enrichment of EGFR, AK
lysates enabled detection
Immunoprecipitation of E
of multiple isoforms and p
EGFR, AKT1, AKT2 and
by nLC-SRM/MS in two c
Enrichment of as low as 7
of recombinant PIK3CA/P
absolute quantitation by t
Mulitplex IP to MS allowe
EGFR, AKT2, AKT1 and
Future work will focus on
abundant targets (<1ng to
References
1. Logue JS, Morrison DK. Co
use of targeted inhibitors in
50.
2. Gingras AC, Gstaiger M, R
using mass spectrometry. N
3. Carr SA, Abbatiello SE, Ack
Biology and Medicine: Best
Development Using a Fit-fo
13(3):907-17.
4. Ackermann BL . Understan
spectrometry methods for c
58(12):1620-2.
Scaffold is a trademark of Proteome Softwar
and its subsidiaries.
For Research use only. Not fo
This information is not intended to encourag
intellectual property rights of others.
FIGURE 9. Summary of EGFR-A
two cell lines without and with
Target
Cell line
De
EGFR
A431
HEK293
AKT1
A431
HEK293
AKT2
A431
HEK293
AKT3
A431
HEK293
Grp94
A431
HEK293
PIK3CA
A431
HEK293
PIK3R1
A431
HEK293
PTEN
A431
HEK293
FIGURE 8. Multiplex immunoprecipitation to MS research applications.
EGFR, AKT isoforms and PTEN were enriched simultaneously from HEK293 lysate with
biotinylated antibodies, captured with Pierce Streptavidin coated magnetic beads. All four
targets were identified and quantified by MS.
Targets/
HEK293 lysate
% Sequence
Coverage
EGFR
17%
AKT2
23%
AKT1
16%
PTEN
11%
nLC-MS/MS
nLC-SRM/MS
Targets/
HEK293 lysate
Concentration
(fmol)
Concentration
(ng)
EGFR
46
6.2
AKT2
96
5.4
AKT1
>ULOQ
>ULOQ
PTEN
89
4.2



















