
3
Thermo Scientific Poster Note
•
PN-64139-ASMS-EN-0614S
/AKT/PTEN proteins for
s spectrometry (IP-MS)
ly coupled antibodies or
n. EGFR, PI3K, AKT isoforms
optimized IP to MS workflow. A
ased MS research method
Q) of EGFR, AKT2, AKT1,
gets (EGFR, AKT isoforms,
ntified by targeted SRM
ulted in overall higher yield of
e beads for MS research
N from two cell lysates
low as 7ng recombinant
ation by LC-SRM. Multiplexed
tification and quantitation by
rs in clinical research is the
ls of proteins of interest in
ctrometry (MS) are
ctive characterization and
es, tissue, and biofluids. IP
S provides high selectivity,
ncentrations in different
on and PTM status of EGFR-
e characterization of the
IP methods (Hydrazide
e bead, NHS-Ester activated
ndirect IP methods (Streptavidin
e™
Streptavidin magnetic
nd in-solution trypsin digestion
enrichment of medium to low
A) for MS applications. Multiplex
targets simultaneously from
Streptavidin coated magnetic
H 8 followed by reduction,
ysis, tryptic digest samples were
Tips. For discovery MS, the
tem at 300 nL/min over a 45
spectrometer (DDA, Top 6,
SRM/MS with the Thermo
mo Scientific™ Easy
nanoLC II
Proteome Discoverer™ 1.4
erage, spectral counts and
ientific ™ Pinpoint™ and
(LOQ ) and target analyte
low abundant proteins.
Results
Benefits of Magnetic Beads for IP-MS
•
Lower background: Minimal non-specific binding
•
High signal to noise: Easy and efficient washing, less void volume reduces the
chance of losing sample
•
Easy handling: Easy separation of resin
•
Time and effort: Less washing and faster incubation (60 minutes start to finish)
•
Better reproducibility: Product and handling consistency
•
Ab savings: All binding on outer surface
•
Automation: Improves throughput and reproducibility
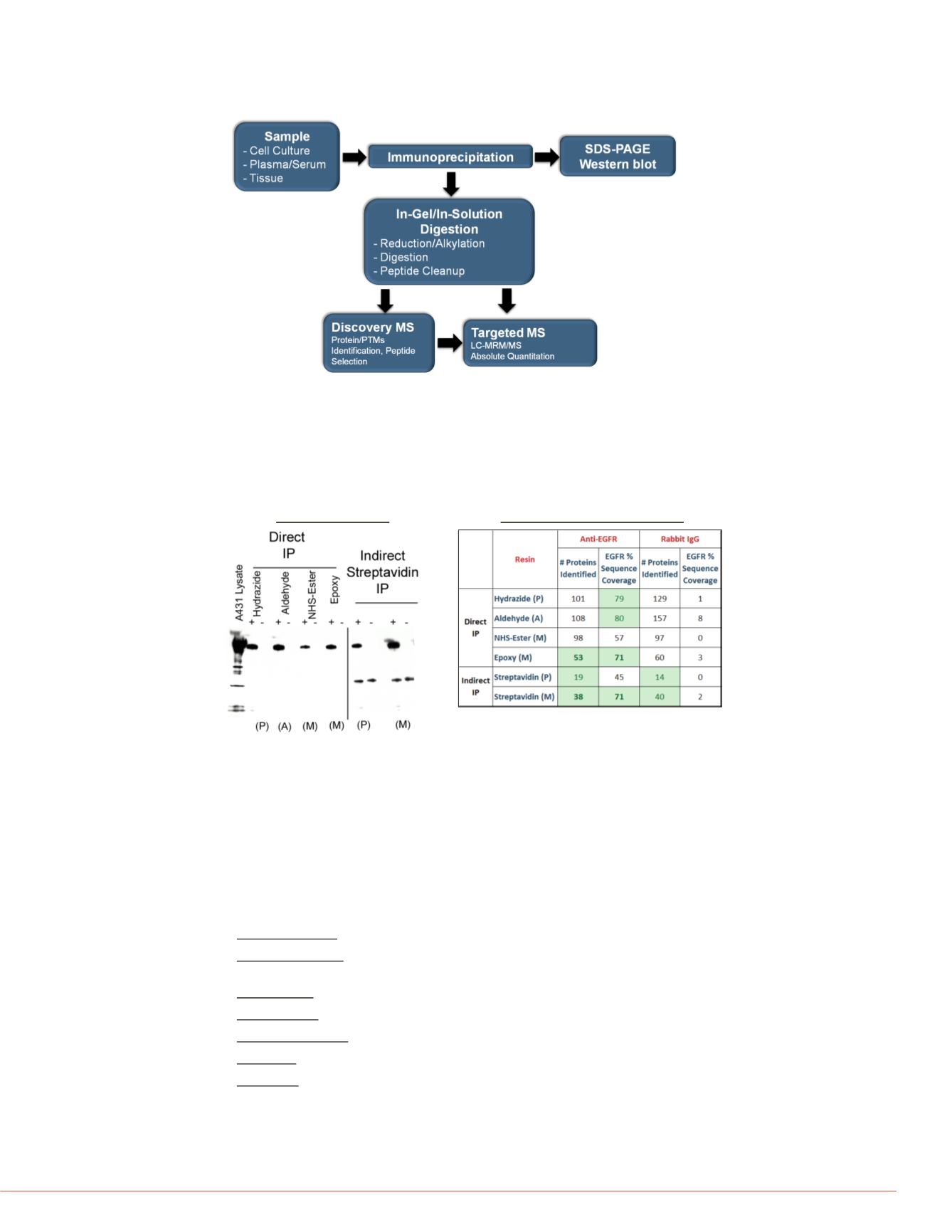
FIGURE 3. Evaluation of EGFR immunocapture efficiency and selectivity.
EGFR immunoprecipitation was used to evaluate directly coupled antibody or
biotinylated antibody with immobilized streptavidin resin. A) Capture efficiency was
determined by Western blot. B) EGFR sequence coverage and background proteins
were determined by LC-MS/MS after elution and trypsin digestion. IP using magnetic
beads resulted in fewer background proteins identified and higher EGFR sequence
coverage.
FIGURE 2. Experimental workflow for IP-MS research method development.
Protein targets are immune-enriched from matrix and analyzed by silver stain or
Western blot after gel electrophoresis. IP samples are also digested with trypsin and
analyzed by nLC-MS/MS to identify candidate quantitative peptides. Heavy isotope-
labeled, quantitative peptide standards are then used in targeted SRM or MRM
research methods for absolute quantitation.
Anti-EGFR Western
EGFR
Heavy
chain
In-Solution nLC-MS/MS Results
Success Criteria: <60 >60%
P: Polyacrylamide, A: Agarose
M: Magnetic
+ Anti-EGFR; - Rabbit IgG
FIGURE 4. Identification of multip
A
B
A) IP-MS allowed simultaneous ana
and AKT2 peptides. B) MS/MS sp
showing phosphothreonine residue
Enrichment of medium to low ab
Streptavidin Coated Magnetic Be
EGFR-AKT pathway targets were i
biotinylated antibodies, captured wit
washed, eluted, digested in-solution
coverage and identify isoform-speci
Target
Anti-
%
Sequence
Coverage
EGFR
6
AKT1
3
AKT2
5
AKT3
8
PTEN
1
PIK3CA
0
AKT2
EGFR
1166-Phosphoserine
451-Phosphothreonine
b₃⁺
342.19
b₄⁺-H₂O
453.25
y₅⁺
600.44
y₄⁺
472.50
y₃⁺
401.40
y₁₃²⁺-P
668.54
y₁₅²⁺-P, y₁₄²⁺
773.56
y₈⁺
882.5
y₇⁺
811.60
y₁₅²⁺
822.53
400
600
800
0
5
10
15
20
25
Intensity [counts] (10^3)
Extracted from: R:\Bhavin\IP-MS\IPMS_5Kits_May2013\EGFR\Batch
ITMS,CID@35.00,z=+2,Monom/z=1057.53271Da,MH+=2114.05
ELV



















