
Conclusions
Positive and negative ion detection of co-eluting drugs
was accomplished in a single chromatographic run
using automated polarity switching. Drugs that
underwent a neutral water loss were further fragmented
using WideBand Activation to provide a diagnostically
rich MS/MS spectrum for structural confirmation.
The compound ketoprofen underwent a prominent,
non-specific neutral loss of formic acid and was further
analyzed using an MS
3
transition. Full-scan MS
n
data
was reprocessed to quantify all 16 compounds by
reconstructed ion chromatograms (RICs), or post-
acquisition MRM, and provided results comparable to
triple quadrupole SRM quantitation. It is possible to
achieve the low % RSD required in quantitation due to
the fast cycle time of the Thermo Scientific LTQ. In the
case of non-specific neutral molecule losses, MS
3
experiments generated diagnostic spectra for
confirmational purposes while providing quantitative
results comparable to the MS/MS data. Results of the
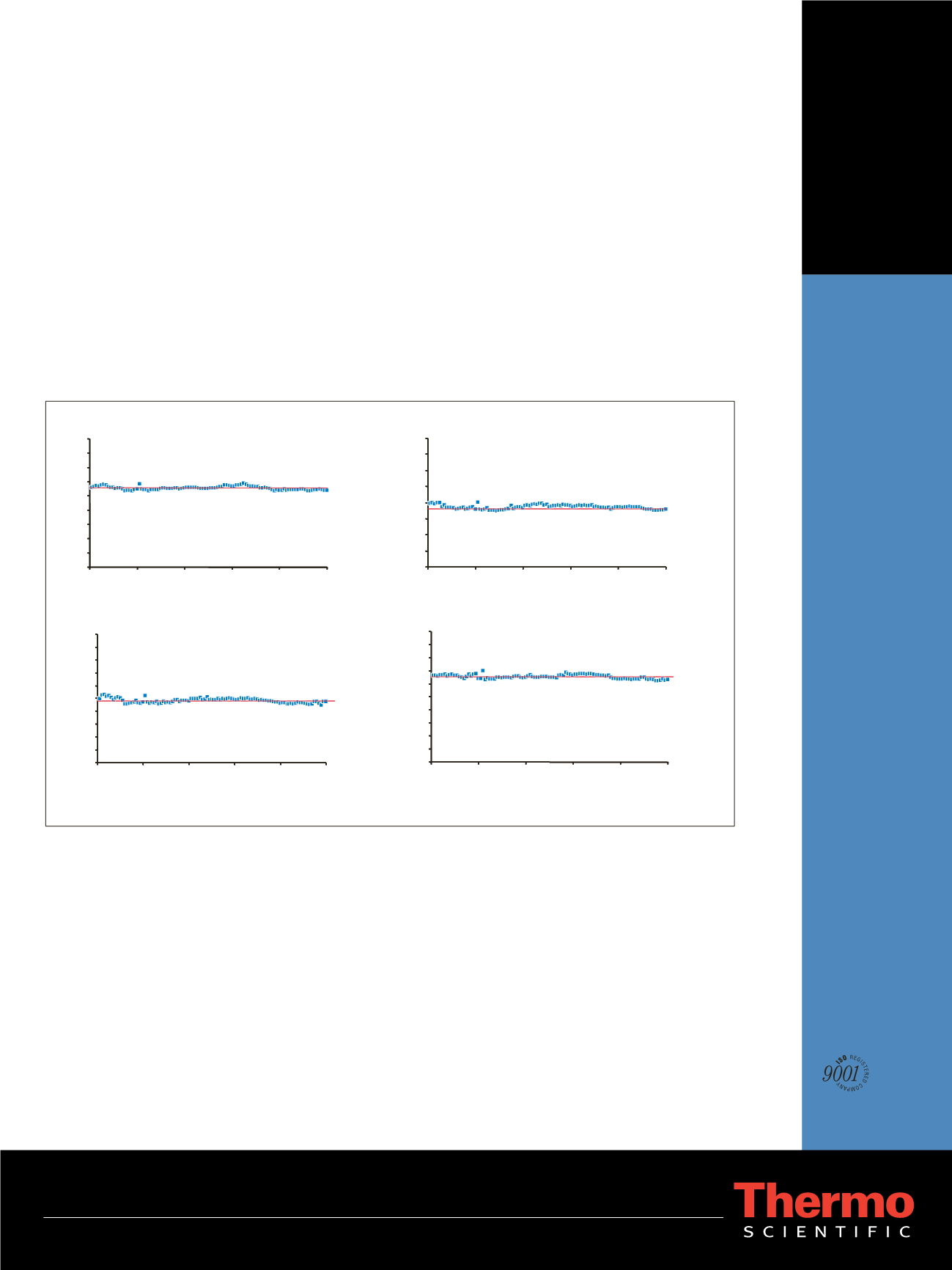
ruggedness study demonstrate no appreciable loss of
sensitivity or reproducibility across 100 replicate urine
injections. Thus, using the Thermo Scientific LTQ
two-dimensional linear ion trap, we have demonstrated
the development of a simple, rapid, and rugged method
capable of confirmational screening and simultaneous
quantitation of drugs in horse urine using both full-scan
LC/MS/MS and MS
3
spectra.
0
100000
200000
300000
400000
500000
600000
700000
800000
900000
1000000
0
20
40
60
80
100
Injection Number
Area
Average Area =482146
CV =3.81%
0
10000
20000
30000
40000
50000
60000
70000
80000
90000
100000
0
20
40
60
80
100
Injection Number
Area
Average Area =65211
CV =2.41%
0
5000
10000
15000
20000
25000
30000
35000
40000
0
20
40
60
80
100
Injection Number
Area
Average Area =18574
CV =3.43%
0
5000
10000
15000
20000
25000
30000
35000
40000
45000
0
20
40
60
80
100
Injection Number
Area
Average Area =27690
CV =2.32%
A
B
C
D
Figure 8: Ruggedness and reproducibility for 100 consecutive injections of a 166 pg/µL standard of theobromine, caffeine, pentoxyphylline,
and ketoprofen in urine
In addition to these
offices, Thermo Fisher
Scientific maintains
a network of represen-
tative organizations
throughout the world.
Africa
+43 1 333 5034 127
Australia
+61 2 8844 9500
Austria
+43 1 333 50340
Belgium
+32 2 482 30 30
Canada
+1 800 530 8447
China
+86 10 5850 3588
Denmark
+45 70 23 62 60
Europe-Other
+43 1 333 5034 127
France
+33 1 60 92 48 00
Germany
+49 6103 408 1014
India
+91 22 6742 9434
Italy
+39 02 950 591
Japan
+81 45 453 9100
Latin America
+1 608 276 5659
Middle East
+43 1 333 5034 127
Netherlands
+31 76 587 98 88
South Africa
+27 11 570 1840
Spain
+34 914 845 965
Sweden/Norway/
Finland
+46 8 556 468 00
Switzerland
+41 61 48784 00
UK
+44 1442 233555
USA
+1 800 532 4752
www.thermo.comAN62535_E 11/07S
Part of Thermo Fisher Scientific
Thermo Fisher Scientific,
San Jose, CA USA is ISO Certified.
Legal Notices
©2007 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. This information
is presented as an example of the capabilities of Thermo Fisher Scientific Inc. products. It is not intended to encourage use of these products in any manners
that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change. Not all products are available in all
countries. Please consult your local sales representative for details.
View additional Thermo Scientific LC/MS application notes at:
www.thermo.com/appnotes


















