
4
Recent findings of long-term metabolites generated from
17-methyl-17-hydroxy-steroids such as metandienone,
oxandrolone, dehydrochloromethyltestosterone, and
oxymetholone, fueled the search for analogous metabolites
in the case of stanozolol. A common feature of the
aforementioned long-term metabolites under ESI-MS/MS
conditions is the elimination of formaldehyde (30 Da),
which also served as indicator in the present study. In the
product ion mass spectrum of the precursor ion [M+H]
+
at
m/z
519, which was attributed to a hydroxylated and
glucuronidated analog of 17-hydroxymethyl,17-methyl-
18-norstanozolol, the ion of the aglycon was observed at
m/z
343.2369 with the experimentally determined elemental
composition of C
21
H
30
O
2
N
2
. In addition, a product ion at
m/z
313.1899 (-30 mass units, Figure 4a) was present.
However, the accurate masses revealed the difference of
C
2
H
6
rather than CH
2
O between
m/z
343 and 313,
demonstrating that the analyzed species was not
17-hydroxymethyl,17-methyl-18-norstanozolol but
16-oxo-stanozolol comprising the same elemental
composition as corroborated by the analysis of the
respective reference substance (Figure 4b). The peculiar
loss of ethane (30 Da) was suggested to originate from the
steroidal D-ring including C-18 and C-20 and the
introduction of a C-13 – C-17 double bond as shown
schematically in Figure 4c. Here, deuterium labeling of
either C-18 or C-20 would provide further insights and
will be subject of future studies.
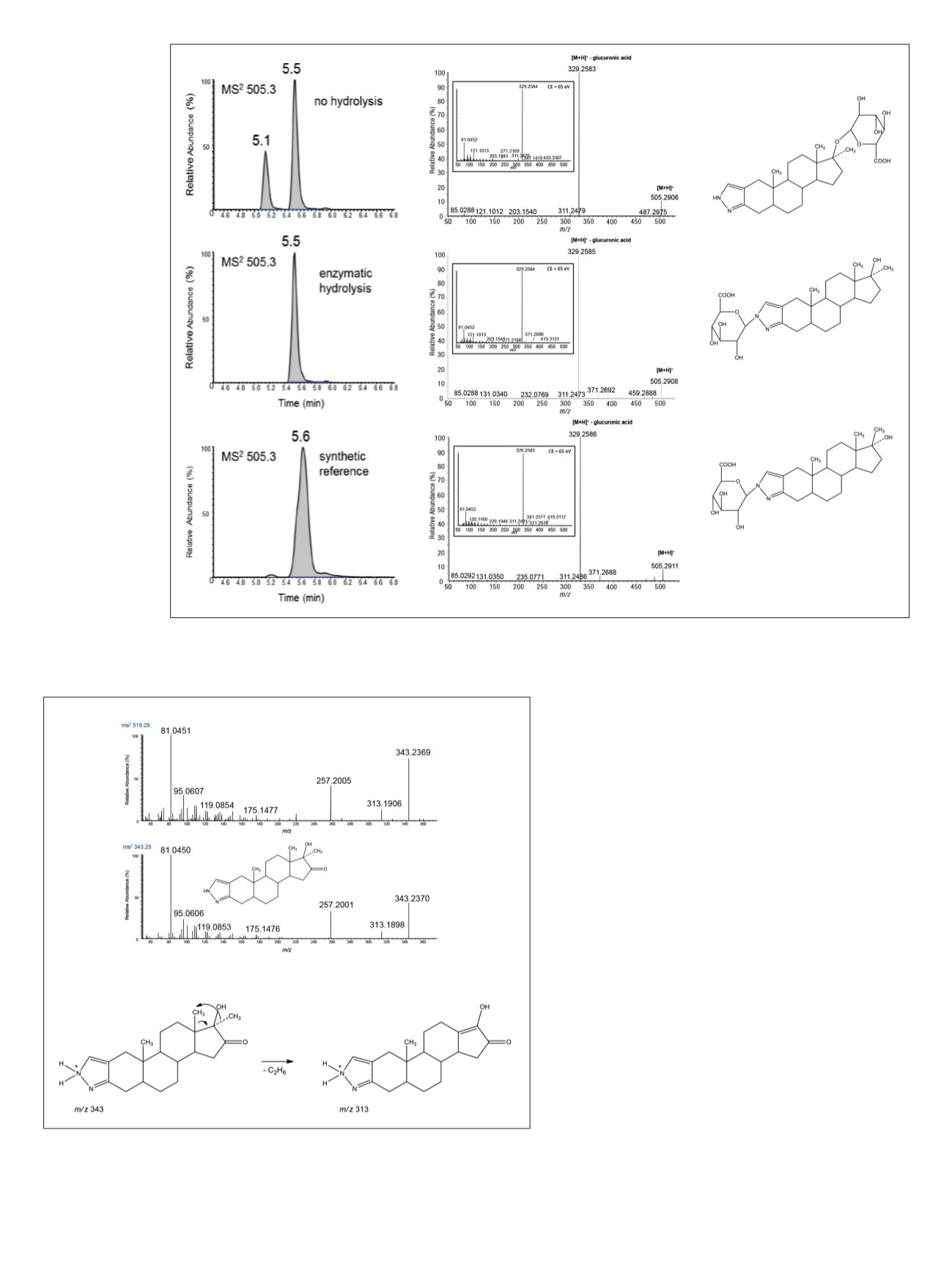
Figure 3. a) Extracted ion chromatograms of a post-administration sample (3 hours after application of 5 mg of stanozolol), indicating the
presence of stanozolol-O-glucuronide (at 5.1 min) and stanozolol-N-glucuronide (at 5.5 min), b) product ion mass spectra of the analytes,
c) structures
Figure 4. Product ion mass spectra of the protonated molecules [M+H]
+
of a) glucuronic
acid conjugate of a metabolite observed in administration study urine samples attributed
to 16-oxo-stanozolol-glucuronide at
m/z
519, and b) reference standard of 16-oxo-
stanozolol. The elimination of 30 Da resulting from the loss of C
2
H
6
is suggested to
include the methyl residues at C-15 and C-17 as illustrated under c).
Stanozolol-O-glucuronide
m/z
504.2835
a)
b)
a)
b)
c)
c)
Stanozolol-N-glucuronide
m/z
504.2835
Stanozolol-N-glucuronide
m/z
504.2835



















