
E1
E2
Dilution
Factor
Expected
(pg/mL)
Measured
(mean, pg/mL)
CV (n=3 %) Accuracy
(n=3, %)
Measured
(mean, pg/mL) CV (n=3, %)
Accuracy
(n=3, %)
256
3.9
3.8
5.0
97.8
3.7
11.7
94.6
128
7.8
8.0
9.0
102.9
8.8
13.9
112.3
64
15.6
16.1
5.1
102.8
15.7
7.4
100.4
32
31.3
32.2
8.4
103.2
29.0
7.6
92.7
16
62.5
59.7
0.8
95.5
62.7
4.4
100.3
8
125.0
123.3
9.9
98.7
129.4
9.8
103.5
4
250.0
245.9
7.0
98.4
253.1
3.7
101.2
2
500.0
503.5
2.3
100.7
478.9
4.1
95.8
1
1000.0
1000.9
4.5
100.1
993.1
5.3
99.3
Mean
100.0
100.0
Validation
The validation procedure included tests for 1) recovery
of sample preparation; 2) lower limit of quantitation
(LLOQ), dynamic range, accuracy; 3) precision;
4) ionization suppression; and 5) carryover.
Results and Discussion
Human plasma has endogenous E1 and E2 so it was not
suitable for validation experiments except the precision
study. Therefore, charcoal stripped serum (CSS) is used to
conduct the validation experiments.
Recovery
The absolute recoveries of E1, E2 and IS from CSS samples
compared to spiked neat solutions ranged from 61.2%
to 65.6%. The relative recoveries of E1 and E2 against
IS ranged from 99.0% to 107.1% at the two spiked
concentration levels (20 and 100 pg/mL).
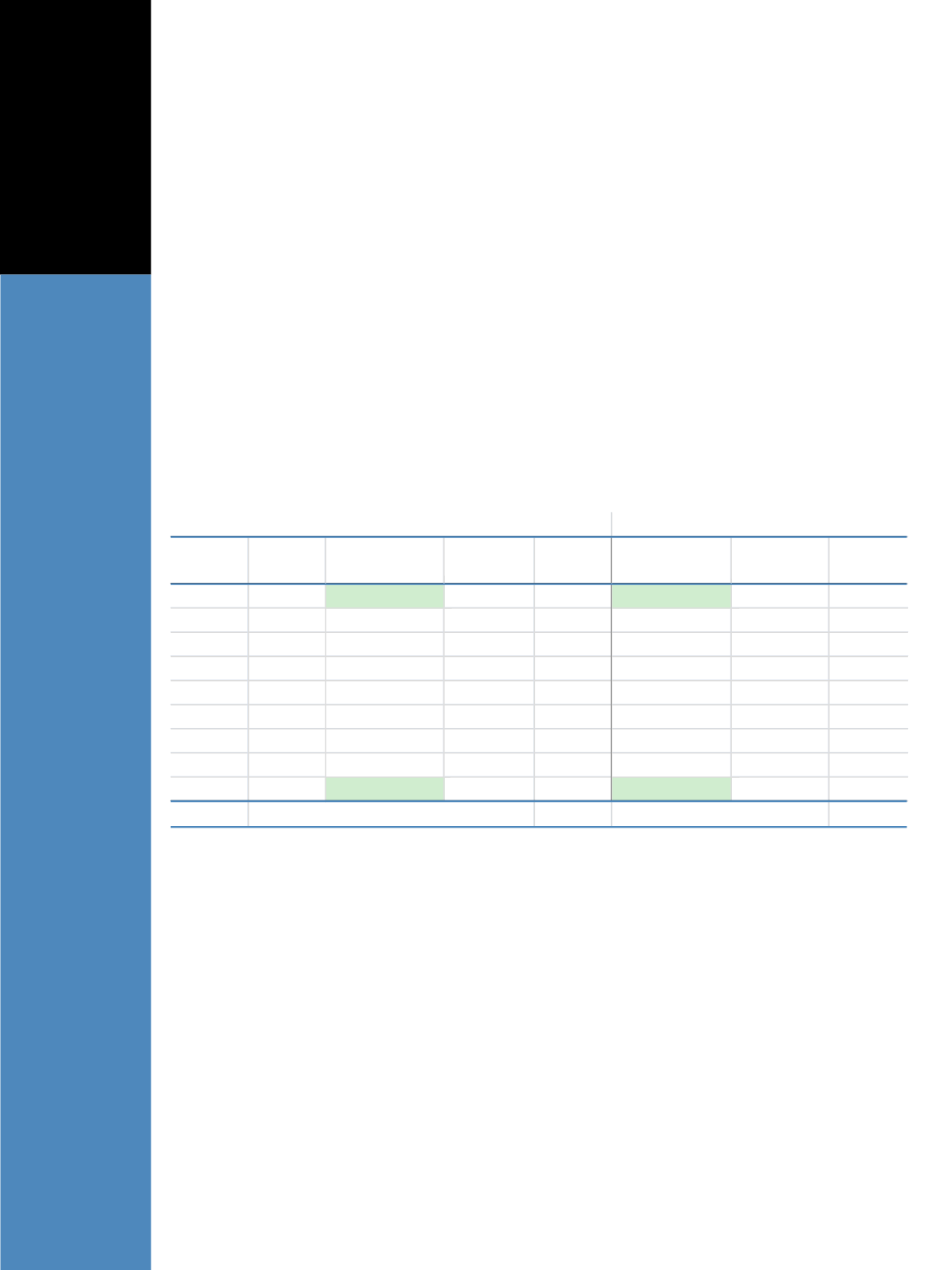
Determination of LLOQ, Linearity and Accuracy
A stock solution of E1 and E2 at 1000 pg/mL was
prepared in CSS. A serial 2-fold dilution with blank
CSS was performed to make 9 levels of linearity samples
with concentrations from 1000 to 3.9 pg/mL for both E1
and E2. Linearity samples were analyzed in triplicate. The
calibration curve was constructed by plotting the
analyte:ISpeak area ratio vs. expected analyte concentration.
The method was linear between 3.8 and 1000.9 pg/mL
with accuracy (n=3) from 95.5% to 103.2% for E1, and
between 3.7 and 993.1 pg/mL with accuracy (n=3) from
92.7% to 112.3% for E2 (Table 1, Figures 2 and 3). The
LLOQ for E1 and E2 are 3.8 and 3.7 pg/mL, respectively
(Table 1 and Figure 4).
Table 1. LLOQ, dynamic range and accuracy



















