
6
Quantitative Confirmatory Analysis of the NIDA 5 Panel Using Prelude SPLC System and TSQ Quantum Ultra MS
Conclusion
An LC-MS/MS method for confirmatory analysis of the 11 drugs in the
NIDA 5 panel using the Prelude SPLC and TSQ Quantum Ultra MSwas
developed and validated.
The method has LOQs that satisfy the SAMSHA cutoff requirements for
these 11 drugs.
No matrix interference were observed.
The method is simple and fast.
Two-channel multiplexing on Prelude SPLC would allow two different
methods multiplexing in two channels and 3 minutes for a sample.
Acknowledgements
We would like to thank Kent Johnson from Pacific Hospital of Long Beach for
supplying the comparison samples.
thod Precision.
ifferent urine lots.
For Clinical Research and Forensic Toxicology use only
All trademarks are the property of Thermo Fisher Scientific and its subsidiaries.
This information is not intended to encourage use of these products in any manners that might infringe the
intellectual property rights of others.
3
4
5
6
98.3
95.8
101
103
101
98.3
98.5
94.9
101
100
102
98.7
97.9
99.3
103
102
102
96.1
105
98.5
97.6
107
109
106
81
83.5
85.6
85.9
104
108
104
105
91.2
93.7
97.8
106
91
90.7
93.9
92.3
102
103
99.7
104
Precision (RSD%)
Inter-method
HQC
LQC
MQC
HQC
<2.86 15.33
3.23
2.32
<5.24
3.65
2.88
3.62
<11.89 5.84
2.83
2.52
<3.26
4.68
3.31
3.46
<16.63
6.2
4.33
3.79
<2.53
1.84
1.8
2.2
<4.33
8.8
3.57
3.63
<4.11
4.69
3.51
3.67
<2.3
8.3
2.5
3.3
<2.2
8.2
4.8
3
<3.68
5.8
3.99
3.77
Concentrations from Prelude SPLC (ng/mL)
Concentrations from Toxicology Lab (ng/mL)
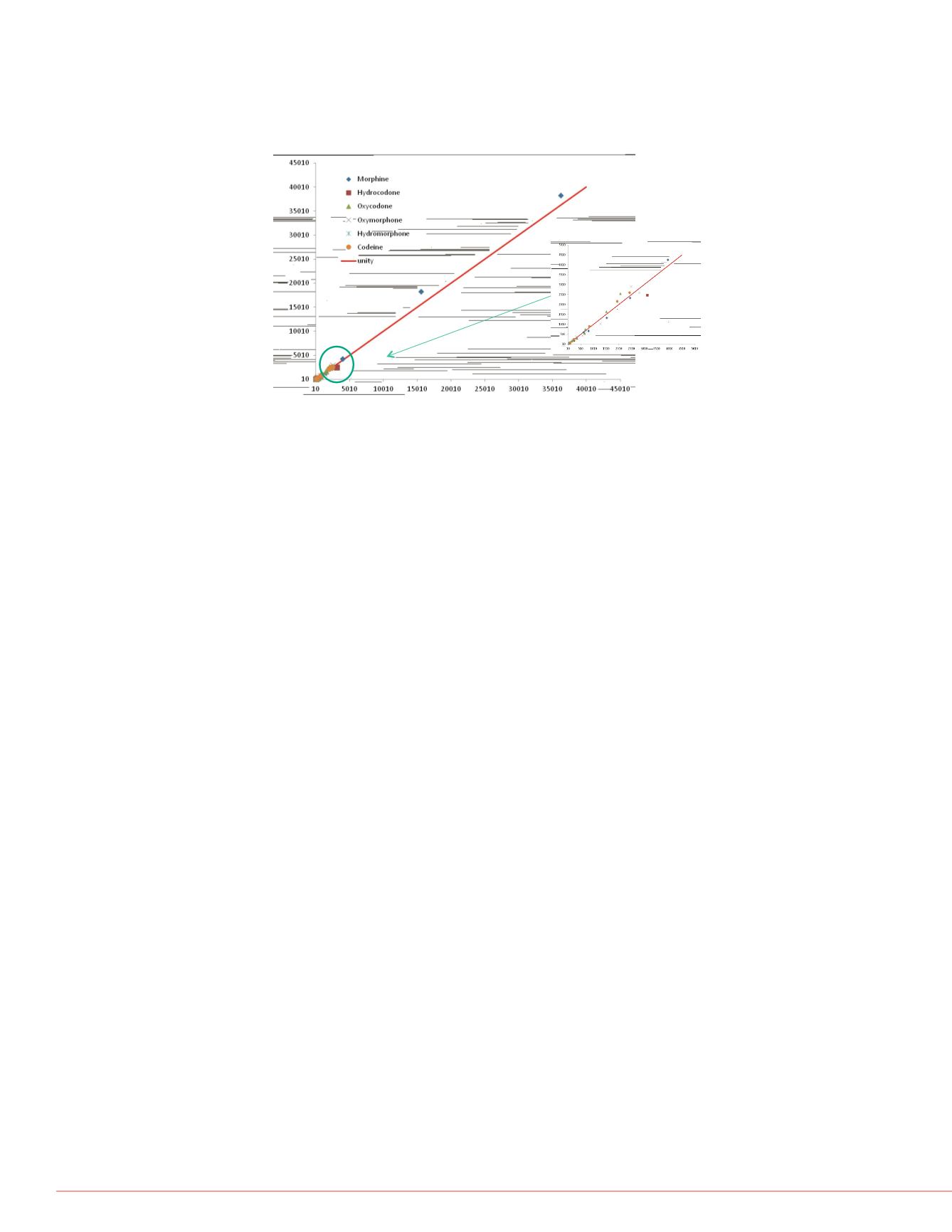
FIGURE 4. Correlation of data acquired with Prelude-Ultra method
compared with data from a toxicology research laboratory validated
method.



















