
5
Thermo Scienti c Poster Note
•
PN ASMS13_M113_SFair_E 07/13S
Conclusion
All 8 compounds show e
system in combination wi
percentages less than 1
these verification analys
Due to the low volume a
SPLC system, these co
less solvent, and with re
The design of the Prelud
up that demonstrates rep
injection time that less th
For research use only. Not for
All trademarks are the property of Ther
encourage use of these products in any
Figure 3. Lower limit of quanititation (LLOQ) for all analytes
.
Table 3. Quality control data
Table 3 shows the resulting qu
and precision. Three consecuti
percentages. All compounds h
showing excellent accuracy an
expected QC value with colum
5) for each day. Then, in colum
standard deviation (SD) in colu
buprenorphine
glucuronide
naltrexone
naloxone
norbuprenorphine
buprenorphine
methadone
norbuprenorphine
glucuronide
EDDP
Analyte
(ng/mL)
buprenorphine
Low QC
Mid QC
High Q
norbuprenorphine
Low QC
Mid QC
High Q
buprenorphine
Low QC
glucuronide
Mid QC
High Q
norbuprenorphine
Low QC
glucuronide
Mid QC
High Q
methadone
Low QC
Mid QC
High Q
EDDP
Low QC
Mid QC
High Q
naloxone
Low QC
Mid QC
High Q
naltrexone
Low QC
Mid QC
High Q
day 2
r
2
day 3
.9976
0.9983
.9969
0.9979
.9982
0.9990
.9993
0.9993
.9976
0.9994
.9991
0.9986
.9942
0.9988
.9924
0.9950
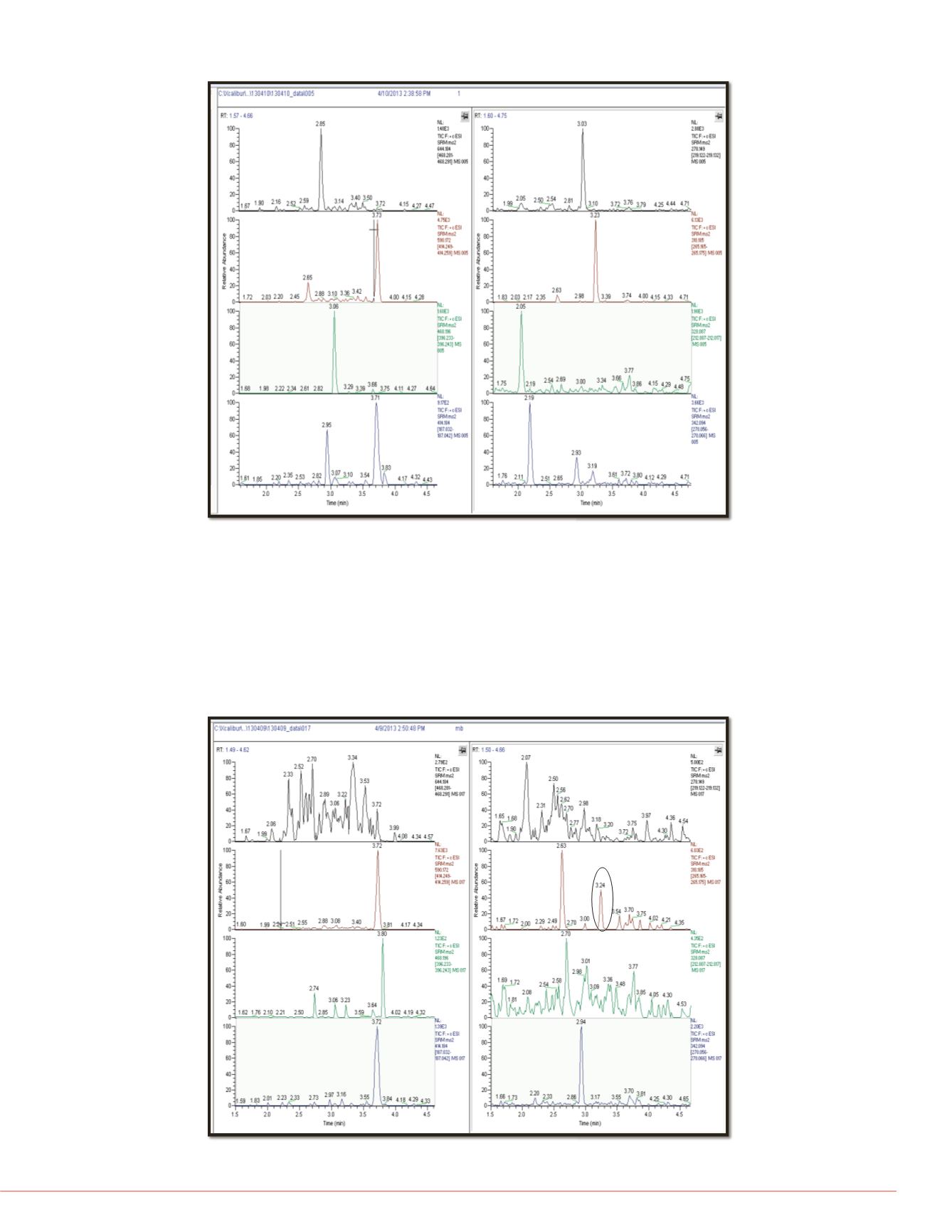
Figure 4 shows the matrix blank that is injected after the highest standard in the
calibration curve often referred to as the upper limit of quantitation (ULOQ). This
matrix blank (n=2) is used to assess the level of carryover for each analyte. The
signal in the matrix blank cannot be greater than 20% of the LLOQ signal. All
analytes have zero carryover at the retention time of interest with one exception:
methadone has an average carryover of about 4.7%, but this is still well within the
allowance of 20% of the LLOQ.
buprenorphine
glucuronide
naltrexone
naloxone
norbuprenorphine
buprenorphine
methadone
norbuprenorphine
glucuronide
EDDP
Figure 4. Carryover as shown in the matrix blanks injected after the ULOQ.



















