
ISO 13485
QMI-SAI Global
Application Note 601
Conclusion
A forensic method for analysis of opiates, opioids, and
their metabolites without hydrolysis has been developed
using the Prelude SPLC system and TSQ Endura MS. By
eliminating the hydrolysis step, the sample preparation
time and analysis cost was drastically reduced. The LC
method on the Prelude SPLC system/TSQ Endura MS
provided ample resolution for all isobaric compounds and
AN64030_E 06/14S
Africa
+43 1 333 50 34 0
Australia
+61 3 9757 4300
Austria
+43 810 282 206
Belgium
+32 53 73 42 41
Canada
+1 800 530 8447
China
800 810 5118
(free call domestic)
400 650 5118
Denmark
+45 70 23 62 60
Europe-Other
+43 1 333 50 34 0
Finland
+358 9 3291 0200
France
+33 1 60 92 48 00
Germany
+49 6103 408 1014
India
+91 22 6742 9494
Italy
+39 02 950 591
Japan
+81 45 453 9100
Latin America
+1 561 688 8700
Middle East
+43 1 333 50 34 0
Netherlands
+31 76 579 55 55
New Zealand
+64 9 980 6700
Norway
+46 8 556 468 00
Russia/CIS
+43 1 333 50 34 0
Singapore
+65 6289 1190
Spain
+34 914 845 965
Sweden
+46 8 556 468 00
Switzerland
+41 61 716 77 00
UK
+44 1442 233555
USA
+1 800 532 4752
www.thermoscientific.com©2014 Thermo Fisher Scientific Inc. All rights reserved. ISO is a registered trademark of the International Organization for Standardization
(Organisation Internationale De Normalization). All other trademarks are the property of Thermo Fisher Scientific and its subsidiaries. This
information is presented as an example of the capabilities of Thermo Fisher Scientific products. It is not intended to encourage use of these
products in any manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change.
Not all products are available in all countries. Please consult your local sales representative for details.
Thermo Fisher Scientific,
San Jose, CA USA is
ISO 13485 Certified.
For Forensic Use Only
an outstanding increase in overall speed of analysis. The
high sensitivity that the TSQ Endura MS provided allowed
for low limits of quantitation of even the least responsive
analytes, like the gluronidated metabolites. The fast SRM
acquisition rate yielded a successful, simultaneous analysis
of 19 compounds with 10 internal standards.
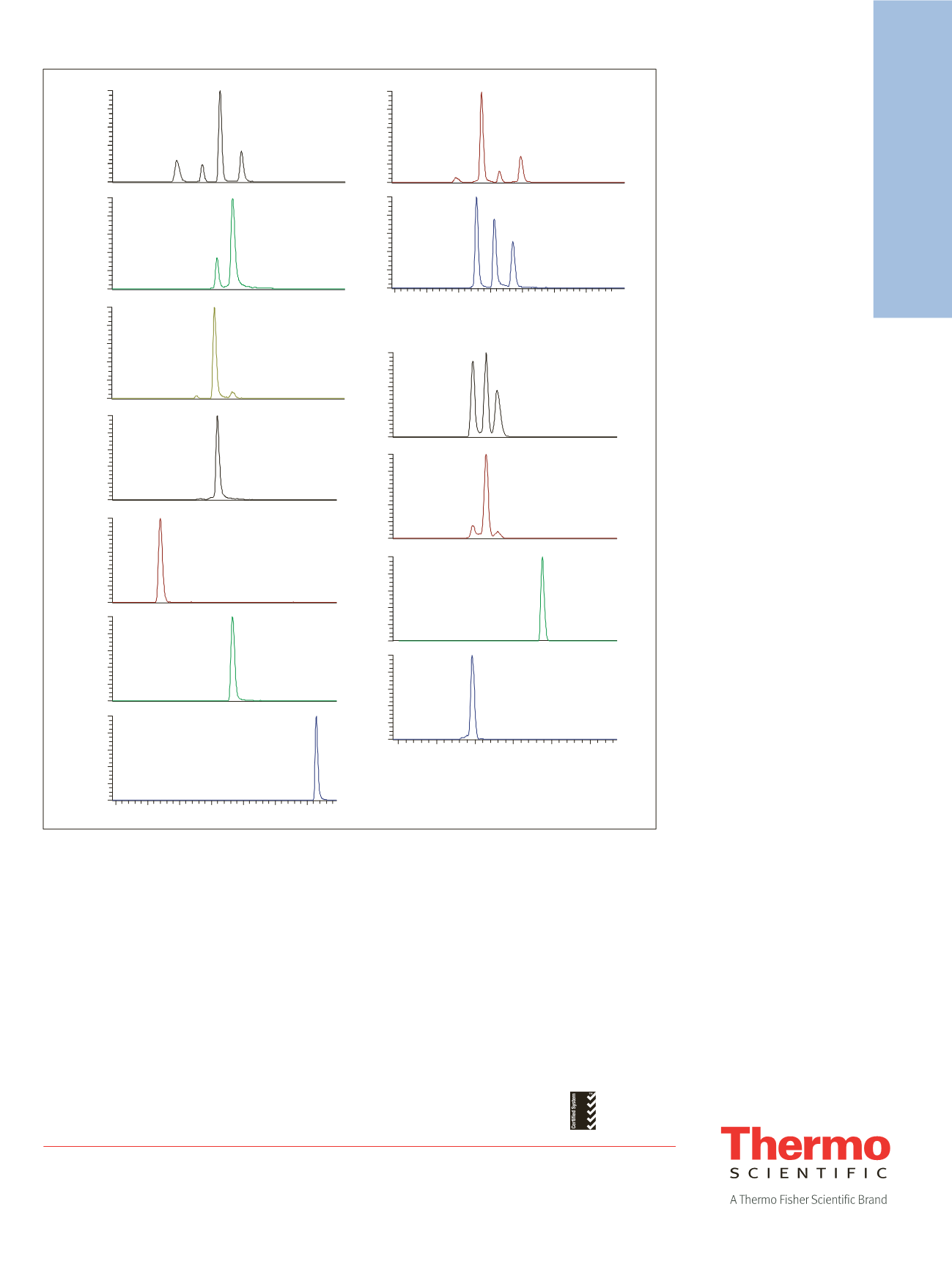
Figure 2. Representative chromatograms for all 19 compounds
0
20
40
60
80
100
Relative Abundance
2.50
2.81
1.86 2.24
Morphine (1.86),
Norcodeine (2.50),
Norhydrocodone (2.81)
0
20
40
60
80
100
Relative Abundance
2.23
2.81
2.50
1.86
Hydromorphone (2.23)
0
20
40
60
80
100
Relative Abundance
2.69
2.45
Codeine (2.45),
Hydrocodone (2.69)
0
20
40
60
80
100
Relative Abundance
2.16
2.42
2.70
Oxymorphone (2.16)
Noroxycodone (2.70)
0
20
40
60
80
100
Relative Abundance
Relative Abundance
Relative Abundance
Relative Abundance
Relative Abundance
Relative Abundance
Relative Abundance
Relative Abundance
Relative Abundance
2.42
2.69
2.15
Dihydrocodeine (2.42)
1.0 1.5 2.0 2.5 3.0 3.5 4.0
Time (min)
0
20
40
60
80
100
0
20
40
60
80
100
0
20
40
60
80
100
0
20
40
60
80
100
2.59
1.70
4.14
0.5
1.0
1.5
2.0
2.5
3.0
Time (min)
0
20
40
60
80
100
0
20
40
60
80
100
0
20
40
60
80
100
0
20
40
60
80
100
1.65
1.47
1.79
1.65
1.46 1.79
2.38
Oxycodone
6-Acetylcodeine
Hydromorphone 3B
glucuronide (1.65)
6-Acetylmorphine
Normorphine
Codeine 6B glucuronide
Morphine 3B
glucuronide (1.47),
Morphine 6B
glucuronide (1.79)
Oxymorphone 3B
glucuronide
1.46
2.83
1.0 1.5 2.0 2.5 3.0 3.5 4.0
Time (min)



















