
2
Table 1. LC gradient
Mass Spectrometry
MS analysis was performed on a Thermo Scientific
™
TSQ
Quantiva
™
triple-stage quadrupole mass spectrometer
(Figure 1). The MS conditions were as follows:
Ionization:
Heated electrospray
ionization (HESI)
Vaporizer temp (°C):
500
Capillary temp (°C):
375
Spray Voltage (V):
800
Sheath gas (AU):
55
Auxiliary gas (AU):
25
Data acquisition mode:
Selected reaction monitoring (SRM)
Chrom filter peak width (s):
3
Collision gas pressure (mTorr):
2
Cycle time (s):
0.2
Q1 (FWMH):
0.7
Q3 (FWMH):
0.7
SRM parameters:
Refer to Table 2
Table 2. Optimized SRM parameters
Method Evaluation
Calibration standards were prepared in charcoal stripped
serum (CSS) (Bioreclamation, LLC) at concentrations of
5, 10, 20, 40, 100, 200, and 500 pg/mL. QC samples
were prepared in CSS at 10 and 50 pg/mL. Intra-assay
precision was obtained by processing and analyzing a
standard curve along with three replicates of each QC
sample. Inter-assay precision was obtained by processing
and analyzing a standard curve along with three replicates
of each QC samples on three different days. Matrix effects
were evaluated by comparing peak areas of a 25 pg/mL
sample prepared in CSS to a sample prepared in
reconstitution solution. Matrix effects in different lots of
plasma were evaluated by comparing the internal
standard signal in donor plasma samples to the internal
standard signal in solvent matrix.
Data Processing
Data was processed with Thermo Scientific
™
TraceFinder
™
software version 3.1. The target ion ratio was calculated
by averaging the values obtained for calibrators and
applying a tolerance of 20% for QC and donor samples.
Results and Discussion
The limit of quantitation (LOQ) was 5 pg/mL, equivalent
to 100 fg on column, with excellent signal-to-noise. The
LOQ was limited by the presence of endogenous
testosterone in CSS (about 1 pg/mL). Figure 2 shows
chromatograms for testosterone quantifier and qualifier
ions at a concentration of 5 pg/mL in CSS. The calibration
range is 5–500 pg/mL. Figure 3 shows a representative
calibration curve. Intra-assay precision was better than
3.4% RSD for the 10 pg/mL QC and 2.0% RSD for the
50 pg/mL QC (Table 3). Inter-assay precision was 2.4%
and 4.6% RSD for the 10 and 50 pg/mL QCs, respectively.
Matrix effects in CSS were not observed. The average
percentage recovery calculated against the spiked solvent
was 94.8%. Limited matrix effects were observed in
donor plasma. Internal standard signal in donor plasma
was about 30% lower when compared to signal in solvent
samples. Ion ratios passed for all calibration standards,
QCs, and donor samples. Figures 4 and 5 present a
TraceFinder chromatogram and calculated ion ratio for
selected donor samples obtained in separate analytical
runs.
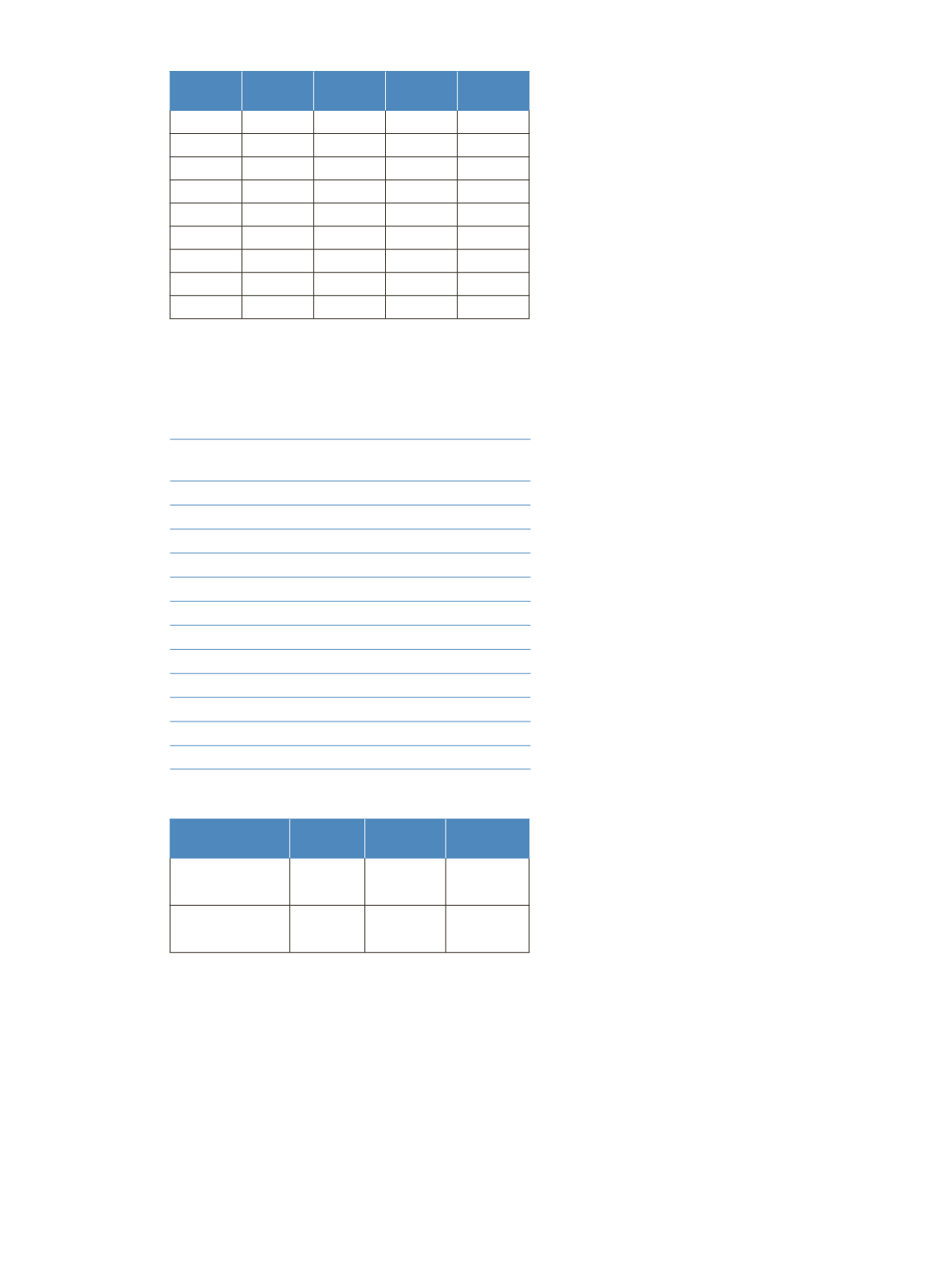
Time
(min)
A
(%)
B
(%)
C
(%)
Flow Rate
(µL/min)
0.00
95
5
0
400
0.10
60
40
0
400
3.60
20
80
0
400
3.61
0
100
0
400
4.60
0
100
0
400
4.61
0
0
100
800
5.00
0
0
100
800
5.01
95
5
0
600
6.50
95
5
0
600
Analyte
Q1
(
m/z
)
Q3
(
m/z
)
CE
(V)
Testosterone
289.1
97.1
109.1
30
30
Testosterone-D
3
292.1
97.1
109.1
30
30



















