
Research Analysis of Clozapine and
Norclozapine in Plasma Using Automated
Sample Preparation and LC-MS/MS
Phillip Morgan, Kings College Hospital NHS Foundation Trust, London, UK
Shane McDonnell, Sarah Robinson, Thermo Fisher Scientific, Hemel Hempstead, UK
Introduction
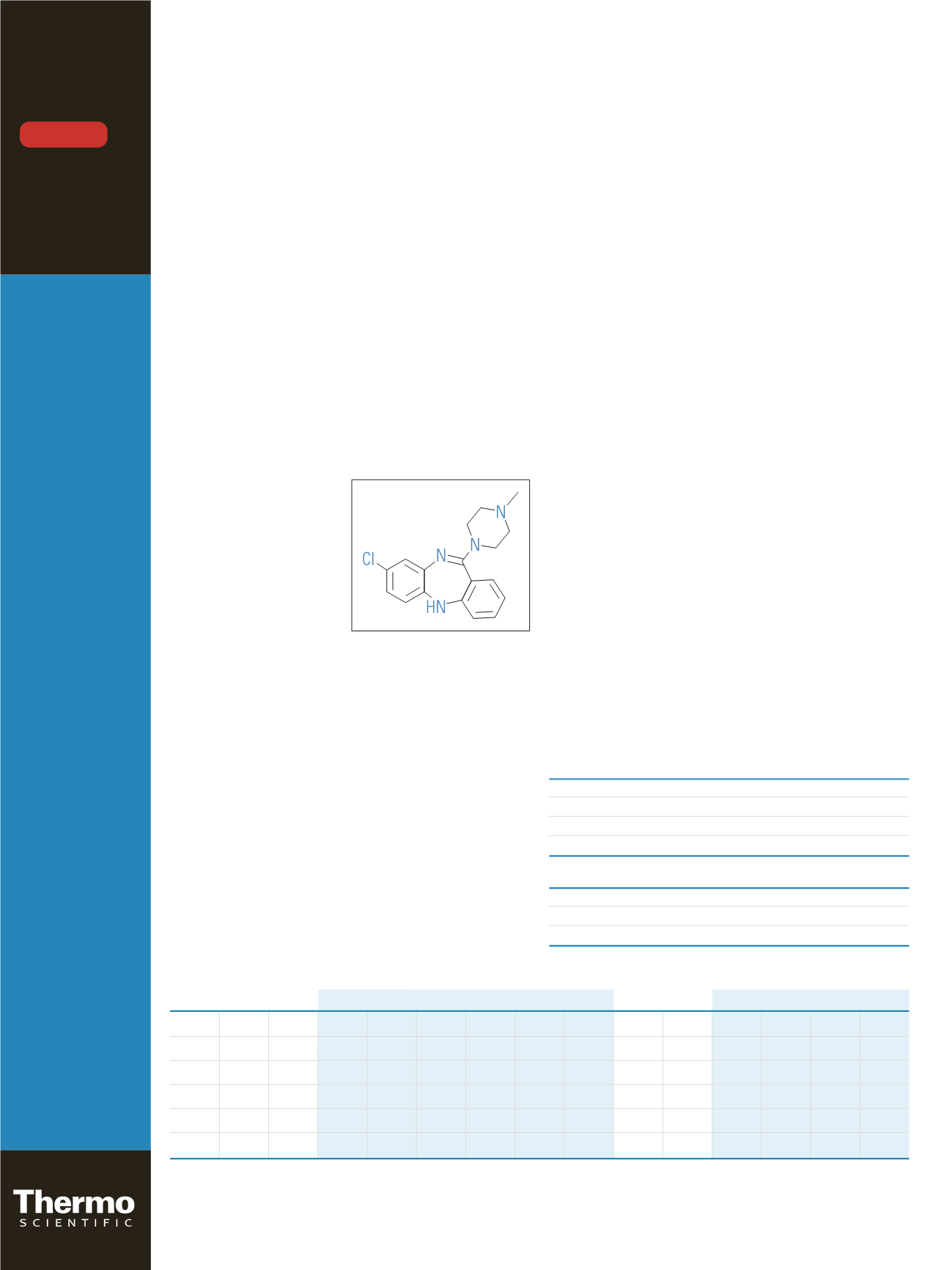
Clozapine (Figure 1) is a tricyclic dibenzodiazepine drug
used in the treatment of schizophrenia. It is uniquely
effective in patients resistant to therapy with other
antipsychotics. In addition to mandatory hematological
monitoring to minimize the risk of agranulocytosis, there
are large variations (50-fold) among patients’ clozapine
dose requirements. Moreover, changes in smoking habits
can have a large effect on the clozapine dose requirement
(on average, the clozapine dose for non-smokers is half
that required for smokers)
due to the induction of
cytochrome P450 (CYP)
enzymes in smokers.
1
Studies
have indicated that accurate
quantification of clozapine
levels may help researchers
better understand, and
conduct analysis of, issues
related to dose optimization
and adherence.
2
Clozapine is metabolized via
N
-demethylation,
N
-oxidation, and aromatic hydroxylation, amongst other
pathways. A few drugs, notably fluvoxamine, block all
four CYP enzymes that can metabolise clozapine.
Measurement of
N
-desmethylclozapine (norclozapine),
which accumulates in plasma to concentrations similar to
that of clozapine, can give useful information regarding
adherence with medication, sample timing in relation to
the last dose of clozapine and drug-drug interactions, such
as that with fluvoxamine.
Current research methodology in our laboratory for
clozapine and norclozapine involves off-line liquid-liquid
extraction with manual transfer to a high pressure liquid
chromatography-ultra violet (HPLC-UV) system. The
Thermo Scientific Aria TLX-1 System powered by
TurboFlow
™
automated sample preparation technology is
being investigated to simplify sample preparation, reduce
the risk of operator error, improve sample throughput,
and gain further selectivity by utilizing tandem mass
spectrometry.
Goal
To assess Thermo Scientific TurboFlow automated sample
preparation technology with tandem mass spectrometry
for the research analysis of clozapine and norclozapine
levels in plasma samples.
Experimental
Sample Preparation
Calibration standards (n=6) were prepared in the range
0.05 mg/L to 2 mg/L by addition of clozapine and
norclozapine to newborn calf serum. Similarly, both
analytes were added to drug-free human plasma to give
internal quality control (IQC) solutions at 0.15, 0.40,
and 1.20 mg/L. After centrifugation at 11,000
g
for
2 min, 10 µL plasma was injected directly onto the
Aria
™
TLX-1 system.
The eluent gradients for both pumps are displayed in Table 1.
TurboFlow LC
Column:
TurboFlow Cyclone 50 x 0.5 mm
Mobile phase A:
0.05% (v/v) aqueous formic acid
Mobile phase B:
0.05% (v/v) formic acid in methanol
Mobile phase D:
45/45/10 Propan-2-ol/acetonitrile/acetone
Analytical LC
Column:
Thermo Scientific Hypersil GOLD C18 50 x 2.1 mm, 3 µm
Mobile phase A:
0.05% (v/v) aqueous formic acid
Mobile phase B:
0.05% (v/v) formic acid in methanol
Key Words
• TurboFlow
Technology
• TSQ Quantum
Ultra
• Clinical Research
Application
Note: 472
Figure 1: Structure of clozapine
Table 1: Gradient programs for both TurboFlow and analytical methods (flow rate is mL/min)
lacitylanA
dohteM wolFobruT
Step Start
Sec Flow Grad %A %B %C %D Tee Loop Flow Grad %A %B
1 00:00 30 1.50 Step 100
-
-
-
==== out
0.50 Step 100
0
2 00:30 60 0.25 Step 100
-
-
-
T
in
0.25 Step 100
0
3 01:30 60 1.50 Step
-
-
-
100 ==== in
0.50 Ramp 5
95
4 02:30 60 1.50 Step 70
30
-
-
==== in
0.50 Step
5
95
5 03:30 60 1.50 Step 100
-
-
-
==== out
0.50 Step 100
0
DOWNLOAD


















