
Sample Preparation
Wastewater samples were filtered using 1.2 µm pore-size
fiber glass filters and then 0.45 µm pore-size mixed cellu-
lose membranes. 50 mM of formic acid and 1 mL of a
5% Na
2
EDTA (w/v) solution were added to 250 mL of
wastewater and the pH adjusted to 3 with NaOH 1.0 M.
Pyrimethamine was used as a surrogate standard and
spiked at a concentration of 500 ng L
-1
.
Analytes were pre-concentrated and extracted using a
200 mg reversed phase polymeric SPE cartridge on top of
a 200 mg mixed mode polymeric SPE cartridge. Retained
analytes were eluted from the cartridges using 2
×
2.5 mL
ACN: MeOH 1:1 (reversed phase) and 2
×
2.5 mL 5%
NH
3
in ACN: MeOH 1:1 (mixed mode). The eluates were
recovered from both cartridges and were collected on the
same conical-bottom centrifuge tube and then evaporated
to dryness with N
2
(g)
. Extracted analytes were reconsti-
tuted to 250 µL with 0.1% formic acid in 90% H
2
O/5%
MeOH/5% ACN solution containing the internal stan-
dards (diaveridine, lomefloxacin and josamycin).
LC-MS/MS Conditions
HPLC separation was done with a Thermo Scientific
Surveyor HPLC system. Detection and quantification of
the analytes was performed with a Thermo Scientific TSQ
Quantum Ultra using the single reaction monitoring mode
(SRM) (Table 1). Two specific single reaction monitoring
(SRM) transitions were used for each compound as well
as their peak area ratios to reliably confirm the presence
of the targeted anti-infectives. This reduced the possibility
of false positives given that some interfering matrix com-
ponents areco-extracted with the analytes and could have
the same SRM transition.
[8]
Results and Discussion
MS/MS in the SRM mode proved to be highly selective.
Instrument response was linear (
r
2
≥
0.99) in the dynamic
range (25–1000 ng L
-1
) in spite of the presence of high
concentrations of organic as well as inorganic interfer-
ences in the matrix. Limits of detection ranged from
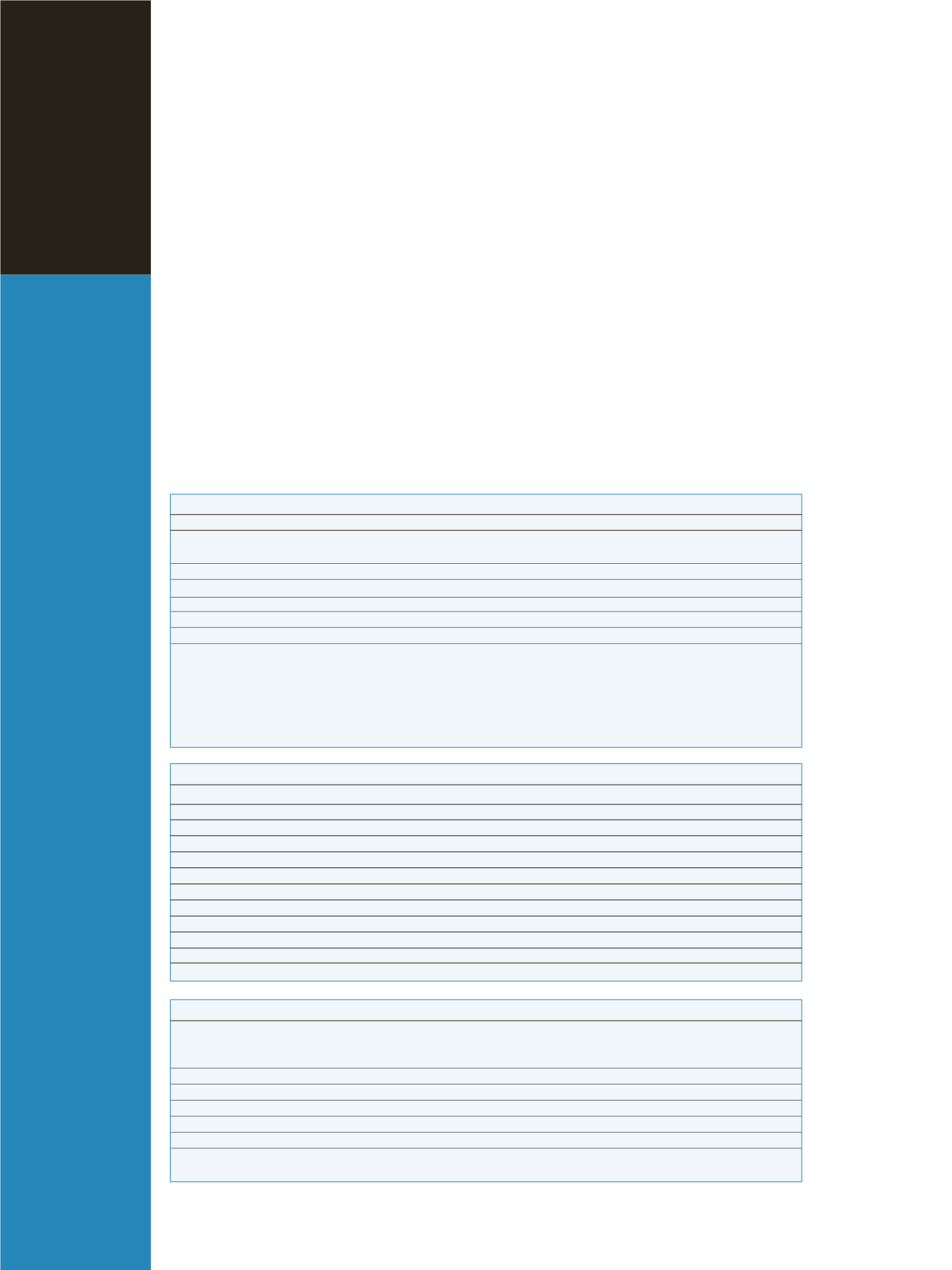
Table 1: Instrument Parameters
HPLC
MS
Column
Thermo Scientific BetaBasic
™
C18
(50
×
2.1 mm, 3 µm)
Ionization mode
ESI+
Column temperature
30°C
Spray voltage
3500 V
Mobile phase A
0.1 % formic acid/H
2
O
Ion transfer capillary temperature
350 ºC
Mobile phase B
0.1% formic acid
/MeOH:ACN1:1
Sheath gas pressure
21 mTorr
Injection volume
20 µL
Auxiliary gas pressure
4 mTorr
Flow rate
200 µLmin
-1
Collision gas pressure
1.5 mTorr
Gradient
t=0 min, A=90%, B=10%
Source CID
–12 V
t=2 min, A=80%, B=20%
t=15 min, A=75%, B=25%
t=17 min, A=50%, B=50%
t=20 min, A=5%, B=95%
t=25 min, A=5%, B=95%
t=30 min, A=90%, B=10%
Table 2: SRM transitions used for detection and quantification (SRM #1) and confirmation (SRM #2)
Compound
SRM #1
CE (V)
SRM #2
CE (V)
Tube Lens
Pyrimethamine
249.10 177.07
40
Sulfamethoxazole
†
254.08 92.11
36
254.08 108.10
37
70
Diaveridine
261.15 123.11
34
Trimethoprim
†
291.16 123.10
33
291.16 230.17
34
91
Ciprofloxacin
‡
332.16 231.07
49
332.16 288.15
27
82
Lomefloxacin
352.17 265.13
34
Levofloxacin
‡
362.17 261.12
35
362.17 221.05
43
92
Clarithromycin*
748.55 590.36
19
748.55 115.99
35
96
Azithromycin*
375.33 82.96
25
749.54 158.04
38
74/112
Josamycin
828.53 108.87
46
828.53 173.96
47
126
†
Quantified using diaveridine as the internal standard,
‡
Quantified using lomefloxacin as the internal standard, *Quantified using josamycin as the internal standard
Table 3: Analytical method parameters
Limit of Detection Standard SRM Sample SRM SRM ratio
Compound
r
2
matrix*
(ngL
-1
)
ratio±SD†
ratio±SD‡
difference^
Sulfamethoxazole
0.9995
22
1.53 ± 0.03
1.6 ± 0.2
-2.6
Trimethroprim
0.9998
7
4.2 ± 0.1
4.39 ± 0.07
-3.3
Ciprofloxacin
0.9996
21
5.5 ± 0.8
6.59 ± 0.05
-18.9
Levofloxacin
0.9996
4
3.65 ± 0.07
3.83 ± 0.06
-5.0
Clarithromycin
0.9997
0.3
1.67 ± 0.04
1.59 ± 0.09
4.3
Azithromycin
0.9900
12
1.2 ± 0.1
0.44 ± 0.1
6.4
*Determination coefficient of the calibration curve made using the WWTP effluent diluted by a factor of 10; **Calculated from the effluent data based on a S/N=3;
†
Standards spiked WWTP effluent diluted by a factor of 10, n=4;
‡
WWTP effluent, n=3;
^
Percentage difference between the standard and sample SRM ratio.



















