Know your analyte for HILIC Method
Development
Knowledge of the compound structure and the physiochemical properties of the molecules under investigation will aid the
choice of column, as it will determine the possible retention mechanisms. Fine-tuning of the separation by optimizing the
organic solvent content, the buffer concentration and the mobile phase pH should follow.
Understanding the physiochemical properties of the analyte is therefore essential to the selection of a suitable HILIC
column.
Firstly, gather information on the hydrophobic character of the analyte, provided by its log P (or log D) value.
The partition coefficient, log P, is used for neutral compounds, or where the compound exists in a single form. Log
P can be calculated from the ratio of concentrations of an un-ionized analyte in the two phases of a mixture of
immiscible solvents (octanol and water being the most commonly used):
Log P indicates the degree of hydrophobicity of a compound. A log P> 0 denotes a relatively hydrophobic
compound; a log P< 0 denotes a more hydrophilic compound.
HILIC is generally recommended for hydrophilic compounds with negative log P values.
Another important analyte parameter is the pK
a
(-log K
a
); K
a
gives us an indication of the acidity of a molecule. K
a
is the equilibrium constant for a chemical reaction and in the context of acid-base reactions is known as dissociation
constant*. The equilibrium can be written symbolically as:
Where HA is a generic acid that dissociates by splitting into:
A
−
, the conjugate base of the acid.
H
+
, the hydrogen ion or proton, which, in the case of aqueous solutions, exists as the solvated proton.
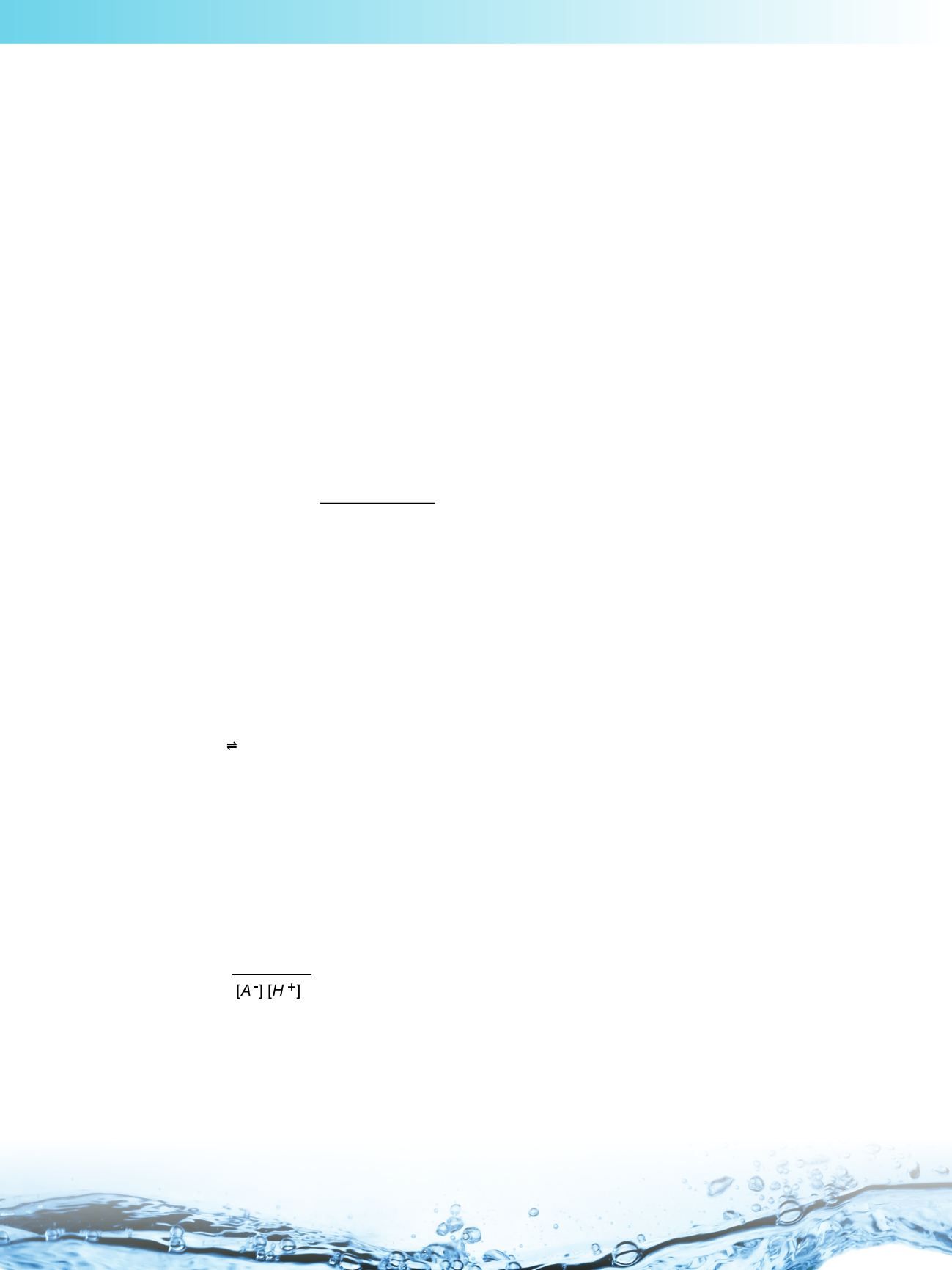
The chemical species HA, A
−
and H
+
are said to be in equilibrium when their concentrations do not change with the
passing of time. The dissociation constant is the ratio of the equilibrium concentrations (in mol/L), denoted by
[HA], [A
−
] and [H
+
]:
The negative logarithmic measure of K
a
is generally used, due to the many orders of magnitude spanned by K
a
values.
12
log P
oct/water
HA A
-
+ H
+
K
a
=
= log
log D
oct/water
= log
B + H
2
O HB
+
+ OH
-
K
b
=
pk
b
14-pk
a
[Analyte]
[Analyte]
[
HA
]
[ionized Analyte]
+
[un
–
ionized Analyte]
[ion zed Analyte]
+
[un
–
ionized Analyte]
octanol
water
≈
water
water
octanol
octanol
[HB
+
] + [OH ]
-
[B]
log P
oct/water
HA A
-
+ H
+
K
a
=
= log
log D
oct/water
= log
B + H
2
O HB
+
+ OH
-
K
b
=
pk
b
14-pk
a
[Analyte]
[Analyte]
[
HA
]
[ionized Analyte]
+
[un
–
ionized Analyte]
[ionized Analyte]
+
[un
–
ionized Analyte]
octanol
water
≈
water
water
octanol
octanol
[HB
+
] + [OH ]
-
[B]
log P
oct/water
HA A
-
+ H
+
K
a
=
= log
log D
oct/water
= log
B + H
2
O HB
+
+ OH
-
K
b
=
[Analyte]
[Analyte]
[
HA
]
[ionized Analyte]
+
[un
–
ionized Analyte]
[ionized Analyte]
+
[un
–
ionized Analyte]
octanol
water
water
water
octanol
octanol
[HB
+
] + [OH ]
-
[B]


