Typical HILIC Stationary Phases
and Applications
In spite of its early beginnings, HILIC did not become widely recognized as a distinct chromatographic mode until it was
'rediscovered' by the scientific community in the early 2000's [8]. The rising popularity of HILIC coincided with a wider
availability of specifically designed HILIC stationary phases with diverse functionalities, which could offer different
selectivity and higher retention for polar compounds [8]. However, unmodified bare or hybrid silica materials are still the
most popular phases. Silica columns specifically intended for HILIC have been developed; these are packed and stored
in aqueous/organic, which contrasts the more conventional use of silica in normal phase chromatography where the
mobile phase would be a mixture of hexane and IPA solvents. Silica materials have also become available in sub-2 µm
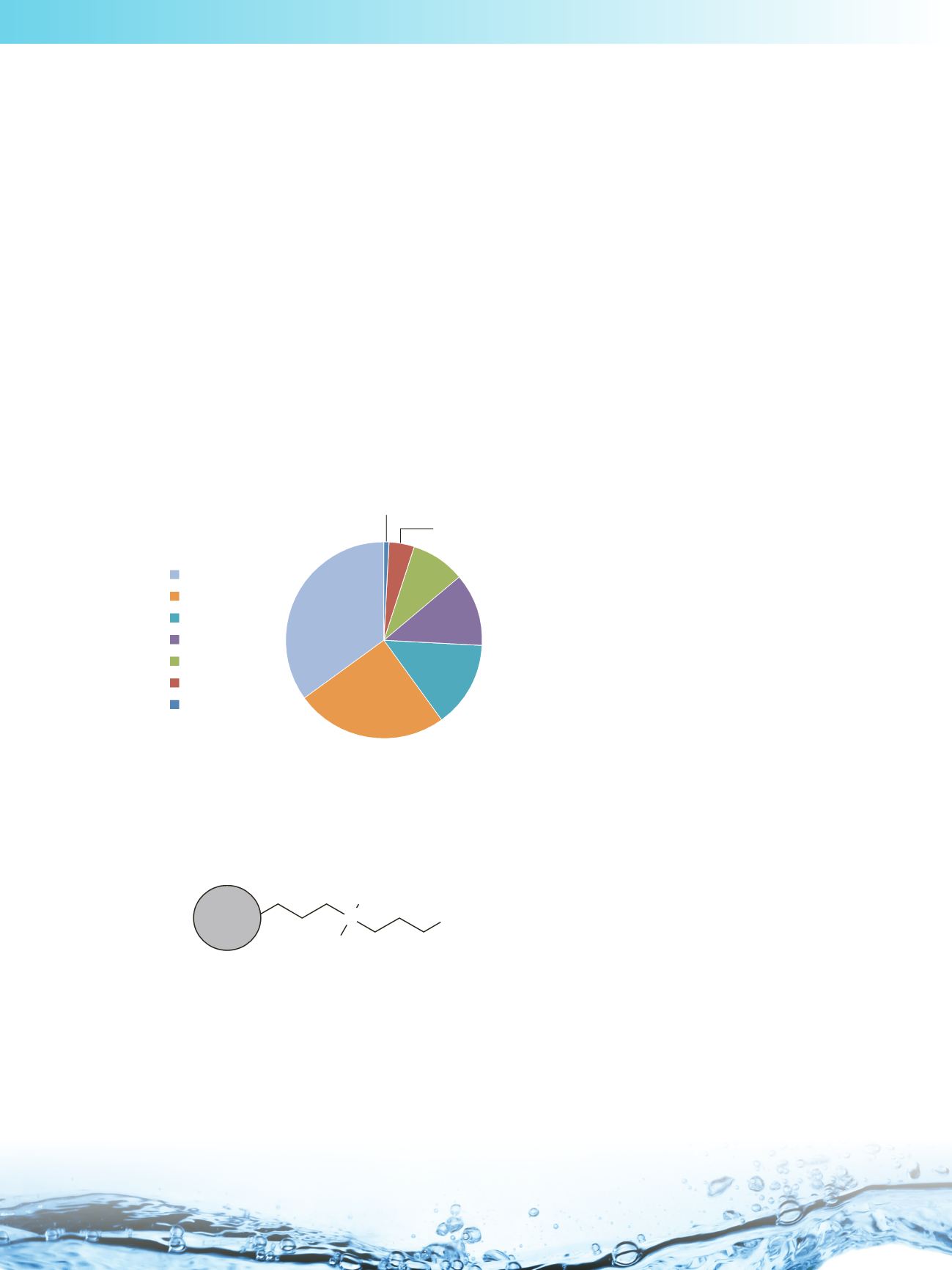
fully porous particles, in superficially porous particles and as monolithic columns. Approximately 35% of the reported
applications are being developed on bare silica, as illustrated below.
HILIC phases vs % of applications
bare/hybrid silica
zwitterionic
amide
diaol
aminopropyl
other
cyanopropyl
35%
9%
25%
14%
12%
1%
4%
Based on Scifinder Scholar 2007 search of the
Chemical Abstracts database 2003−2012
From the data above, it can be seen that the second most employed type of HILIC material are the zwitterionic phases, a
typical example of which is the sulfobetaine phase. The sulfobetaine zwitterion has both positive (quaternary ammonium)
and negative (sulfonic acid) groups in a 1:1 ratio, so that the net surface charge is zero, as illustrated in the figure below:
The sulfoalkylbetaine zwitterionic functionality was originally introduced into polymeric supports by Irgum et al. to prepare
ion-exchange materials for the analysis of inorganic compounds and proteins [1]. Subsequently, a similar functionality
was immobilized on silica substrates. It must be pointed out that, although the net surface charge is zero, the negative
charge of the sulfonic acid at the distal end of the phase may introduce some degree of electrostatic interaction with
charged analytes [9]. Irgum and his group have discussed a new type of zwitterionic phase, with phosphorylcholine
groups grafted onto a polymeric substrate. This material has a positively charged ammonium group at its distal end [10].
Zwitterionic phases are successfully used for the analysis of charged and neutral species, regardless of the possible
electrostatic interactions that can arise.
Sulfobetaine Structure
CH
3
H
3
C
N +
SO
3
-
4


