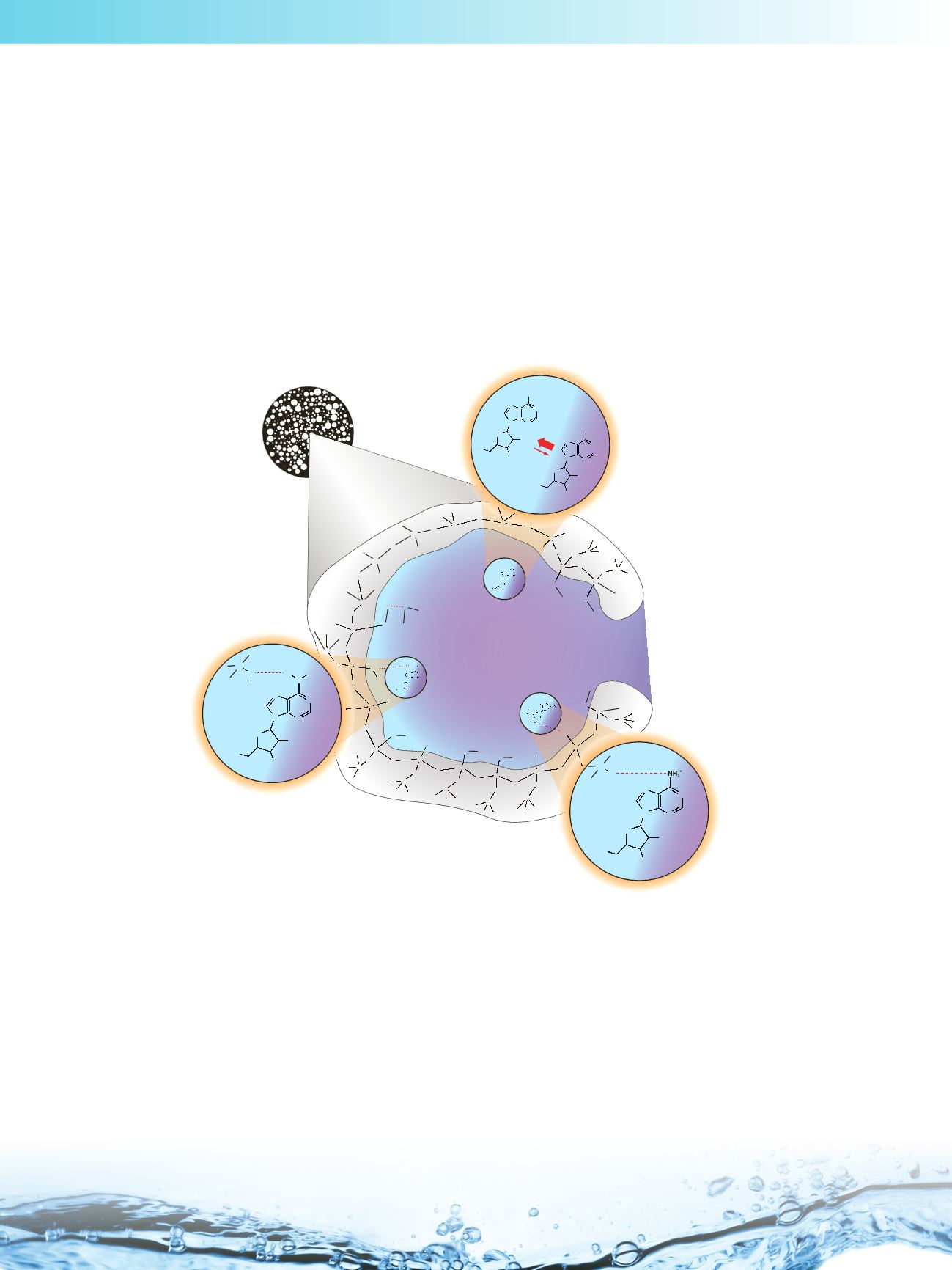
HILIC Retention Mechanism
HILIC can be described as a variation of Reversed Phase Liquid Chromatography (RPLC) performed using a polar
stationary phase. The mobile phase employed in HILIC is highly organic in nature (>60% solvent, typically acetonitrile)
containing a small percentage of aqueous solvent/buffer or other polar solvent. The water in the mobile phase forms an
aqueous-rich layer adsorbed to the polar surface of the stationary phase.
Polar analytes preferentially partition into this aqueous rich layer and evidence [13] suggests that they are retained
through a complex, combination of:
• hydrophilic partitioning of the analyte between the aqueous-rich layer and the bulk of the
mobile phase
• hydrogen bonding between polar functional groups and the stationary phase
• electrostatic interactions on ionized functional groups
Additionally, under the appropriate experimental conditions van der Waals interactions between the hydrophobic portions
of the bonded ligands of the stationary phase (or the siloxane groups, at very low organic solvent concentrations), and the
non-polar part of the analytes can also be present.
H
Y
D
R
O
G
E
N
B
O
N
D
I
N
G
N
O
N
N
N
N
H H
HO
HO
HO
Si
O
H
N
O
N
N
N
HO
HO
HO
Si
O
-
ACN
2
H O
Si
O
O
H
O
O
O
O
O
Si
O
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
O
O
O
O
O O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
Si
O
O
O
O
N
O
N
N
N
N
H H
HO
HO
HO
N
O
N
N
N
HO
HO
HO
H N
H
Y
D
R
O
P
H
I
L
I
C
P
A
R
T
I
T
I
O
N
I
N
G
N
O
N
N
N
HO
HO
HO
N
O
N
N
N
HO
HO
HO
HN
3
+
3
+
NH
3
+
H
H
H
-
H
H
-
H
O
-
H
O
H
H
-
O
N
OH
HO
HO
NH
3
+
N
N
N
Si
Si
O
O
O
E
L
E
C
T
R
O
S
T
A
T
I
C
I
N
T
E
R
A
C
T
I
O
N
6


