Thermo Scientific
| Reagents, Solvents and Accessories
31
FDAA, Marfey’s Reagent
Makes it quick and easy for you to separate and quantitate
optical isomers of amino acids by reverse-phase HPLC.
Optical isomers of amino acids can be simply and
conveniently derivatized with Thermo Scientific FDAA,
Marfey’s Reagent (1-fluoro-2,4-dinitrophenyl-5-L-alanine
amide) – and preparation is complete in just 90 minutes.
With Marfey’s Reagent, the amino acid derivatives can
easily be separated and quantitated by reverse-phase
HPLC. Derivatives have an absorption coefficient of ~3 x 10
4
and can be detected by UV at 340 nm with picomole
sensitivity.
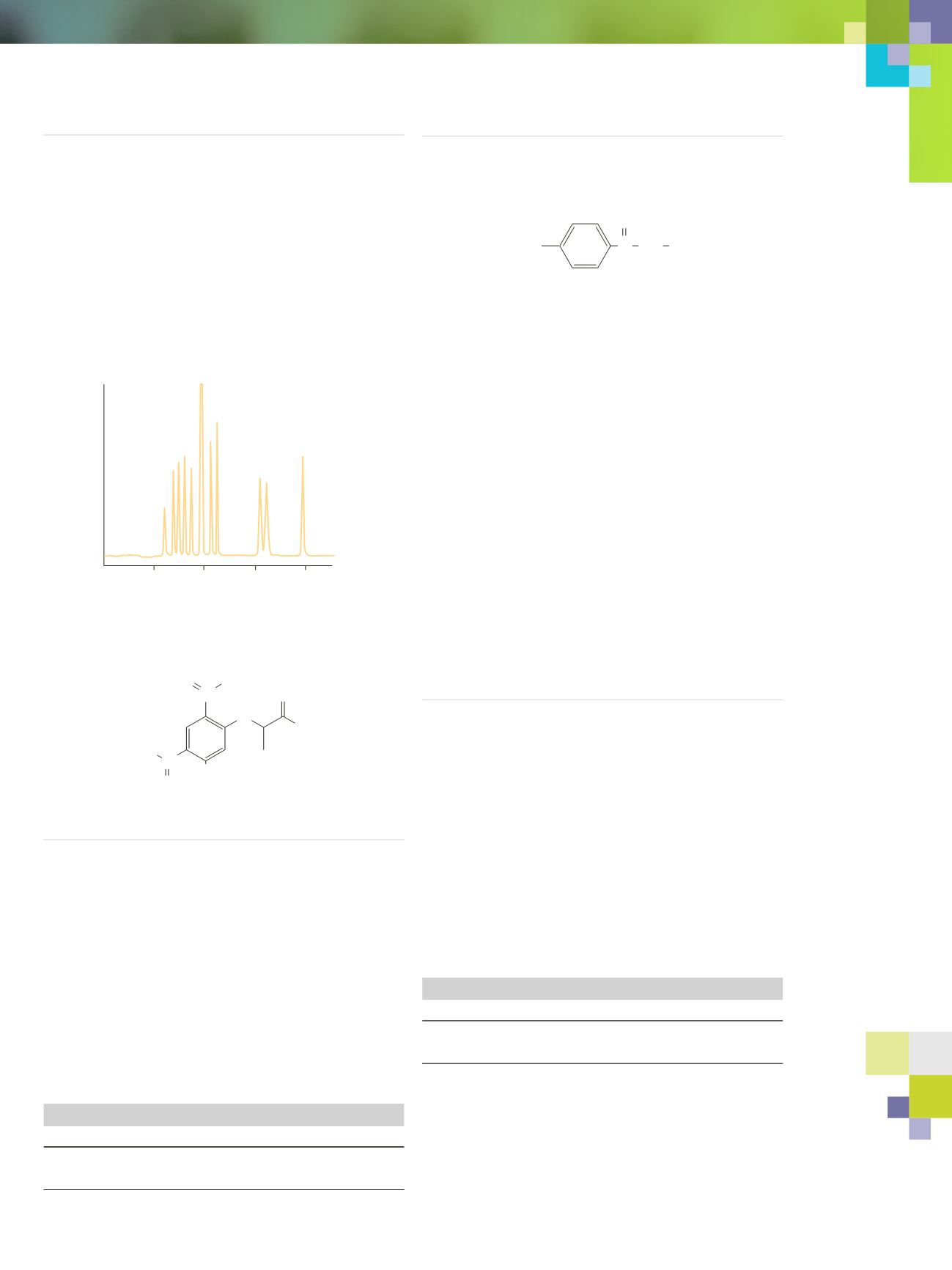
10
20
30 40
Absorbance @ 340 nm
L-Aspartic Acid
L-Glutamic Acid
D-Aspartic Acid
D-Glutamic Acid
L-Alanine Excess Reagent
D-Alanine
L-Methionine
D-Methionine
L-Phenylalanine
D-Phenyalanine
Figure 2. Separation of D- and L-amino acids on 100 mm x 4.6 mm
C18.
Conditions: A) 0.05 M triethylamine phosphate, pH 3.0;
B) acetonitrile. Linear gradient: 10 to 40%B in 45 minutes, 2.0 ml/minute,
25˚C, 340 nm.
PROTOCOL
Preparation of FDAA Derivatives
1. Place 100 µl (5 µmol) sample in a 1.0 ml Thermo Scientific Reacti-Vial
Small Reaction Vial.
2. Add 200 µl of a 1% (w/v) solution of FDAA in acetone. Add 40 µl of 1.0
M sodium bicarbonate. µmol FDAA: µmol amino acid should be
1.5:1.0.
3. Heat at 40˚C for 1 hour. Remove and cool.
4. Add 20 µl 2 M HCI. Allow sample to degas.
5. Analyze. Conditions: 100 mm x 4.6 mm C18; UV at 340 nm
A: 0.05 M TEA phosphate, pH 3.0; B: CH3CN
Linear gradient: 10% B to 40% in 45 minutes, Flow: 2.0 mI/minute at
25˚C
Reference
1. Marfey, P. (1984).
Carlsberg Res. Comm.
49,
591-596.
Ordering Information
Product # Description
Pkg. Size
TS-48895
FDAA, Marfey’s Reagent
50 mg
(1-fluoro-2,4-dinitrophenyl-5-L
-alanine-amide)
p
-Bromophenacylate Reagent
Procedure gives quantitative yields with few or no side
reactions.
C
O
CH
2
Br
Br
p-Bromophenacylate
MW 277.94
Durst,
et al.
have described a novel preparation of various
phenacyl esters and their use as UV visualizing agents in
the 1-10 ng range. This procedure gives quantitative yields
with few or no side reactions. Phenacyl esters have been
used to separate many saturated and unsaturated fatty
acids,
2,3
including prostaglandins.
4
Phenacyl esters have some significant advantages over
previously reported methods, including:
• Pre-mixing of phenacylbromide and crown ether is not
necessary
• Derivatization is both rapid and quantitative, with yields of
more than 95% in 15-20 minutes at 80˚C
• Excess reactants do not interfere
• Large excess of alkylating reagent is not necessary
• Small amounts of water or alcohol do not interfere
• If isolation is desired, products usually are crystalline
PROTOCOL
Preparation of Phenacyl Esters
p
-Bromophenacylate Reagent
(0.1 µmol/ml
p
-Bromophenacylbromide,
0.005 µmol/ml crown ether in acetonitrile)
1. Dissolve ~10 mg acid in MeOH in a 5.0 ml Thermo Scientific Reacti-Vial
Small Reaction Vial fitted with Thermo Scientific Reacti-Vial Magnetic
Stirrer. Neutralize to the phenolphthalein endpoint with KOH/MeOH.*
2. Evaporate the MeOH with N
2
.
3. Add 1.0 ml Phenacylate Reagent and 2.0 ml dry CH
3
CN.
4. Heat at 80˚C with stirring for 30 minutes.
5. Remove and cool.
6. Analyze. Conditions: C18; UV at 250 nm
A: CH
3
CN; B: deionized H
2
0
Linear gradient: 80% A to 100% A; Flow: 2.0 mI/minute
* If the formation of potassium salts is undesirable, neutralize by adding
KHCO
3
at five times the total acid instead of using KOH.
Ordering Information
Product # Description
Pkg. Size
TS-48891
p-Bromophenacylate Reagent
10 ml Hypo-Vial
0.1 mmol/mI p-Bromophenacylbromide,
Sample
0.005 mmol/ml crown ether in acetonitrile
Storage Vial
H
N
NH
2
O
F
N
+
–
O
O
N
+
O
–
O
FDAA
(Marfey's Reagent)
MW 272.19
H
N
NH
2
O
F
N
+
–
O
O
N
+
O
–
O
FDAA
(Marfey's Reagent)
MW 272.19


