Thermo Scientific
| Reagents, Solvents and Accessories
27
Thermo Scientific
HPLC Ion Pair Reagents
Triethylamine (TEA)
Ideal for HPLC separation and analysis of peptides!
Triethylamine is an ion-pairing reagent that alters selectivity
in reverse-phase HPLC separations. By pairing with
peptides, it effectively sharpens peaks, resulting in improved
peak resolution. Triethylamine comes in two grades. Our
ionate grade is designed for use as an ion-pair reagent in
HPLC separations and has a low UV absorbance to provide
you with the most sensitive detection across all
wavelengths. Sequanal grade is designed to meet the
special requirements for peptide sequencing and analysis.
Highlights:
• > 99.5% triethylamine purity, allowing sensitive peptide
detection at low UV wavelengths in reverse-phase HPLC
peptide separation systems
• Packaged in amber glass bottles with protective Teflon
®
TFE-lined fluorocarbon caps for reagent integrity
Ordering Information
Product # Description
Pkg. Size
TS-53101
Triethylamine (TEA), HPLC Grade
25 g
✖
TS-25108
Triethylamine (TEA), Sequencing
100 g
Grade
✖
Additional hazardous handling charge.
Formic Acid Ampules
Ideal reagent for LC-MS applications.
High Purity Thermo Scientific Formic Acid is sealed in
amber glass ampules under a dry nitrogen atmosphere.
A pre-measured aliquot of acid greatly simplifies the
preparation of liter quantities of mobile phases at the
standard 0.1% formic acid concentration. The quality of the
formic acid coupled with ampule packaging provides
reliability and convenience that adds value to both the
chromatographic and MS results.
Formic Acid, Reverse-Phase HPLC and Mass Spectrometry
Formic acid is a component commonly found in
reverse-phase mobile phases to provide protons for LC/MS
analysis. The presence of a low concentration of formic
acid in the mobile phase is also known to improve the peak
shapes of the resulting separation. Unlike trifluoroacetic
acid (TFA), formic acid is not an ion-pairing agent and it
does not suppress MS ionization of polypeptides when used
as a mobile-phase component.
Highlights:
99% pure formic acid
• Consistent LC baselines
• No potential interference introduced in LC
or MS applications
• No signal suppression in the mass
spectrometer
High-performance ampule packaging
• Amber glass, pre-scored, nitrogen-flushed ampules
protect formic acid from light and moisture
Convenient format
• Ampule packaging simplifies the preparation of gradient
and isocratic mobile phases containing 0.1% (v/v) formic
acid in water or acetonitrile; the contents of a single vial in
a final volume of 1 L of solvent yields a mobile phase of the
most common formic acid concentration.
Ordering Information
Product # Description
Pkg. Size
TS-28905
Formic Acid 99+%
10 x 1 ml ampules
0 Min 3
0 Min 3
0 Min 3
0 Min 3
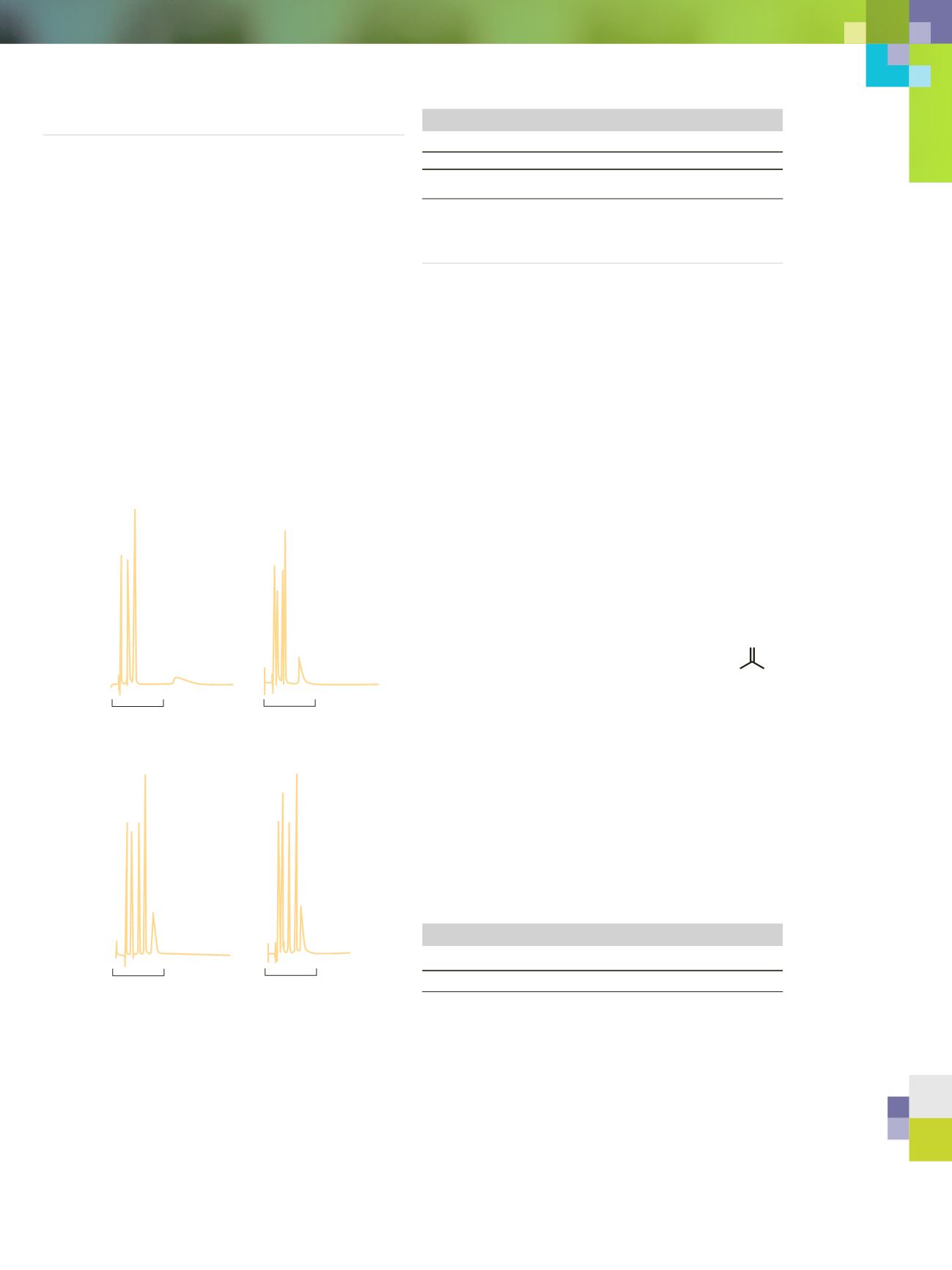
2(a);0.000M TEA 2(b);0.010M TEA 2(c);0.020M TEA 2(d);0.030M TEA
2,4-dimethylaniline
2,4-dimethylaniline
2,4-dimethylaniline
2,4-dimethylaniline
Figure 1A-1D. Effect of TEA concentration on a mixture of basic
antihistamines and 2,4-dimethylaniline* 150 mm x 4.6mm C8.
Conditions: a) 40% methanol, 0.060 M HSA sodium salt, 0.045 M citric
acid; b) 0.150 M citric acid, 0.060 M TEA, pH 7.5 with NaOH; c) 0.150
M citric acid, pH 7.5 with NaOH; isocratic with TEA concentrations
modified by varying b/c ratio, 3 min./ml, 50˚C, 254 nm.
Formic Acid
MW 46.03
H HO
O
3
0 Min 3
0 Min 3
TEA 2(c);0.020M TEA 2(d);0.030M TEA
2,4-dimethylaniline
2,4-dimethylaniline


