20
For more information, or to download product instructions,
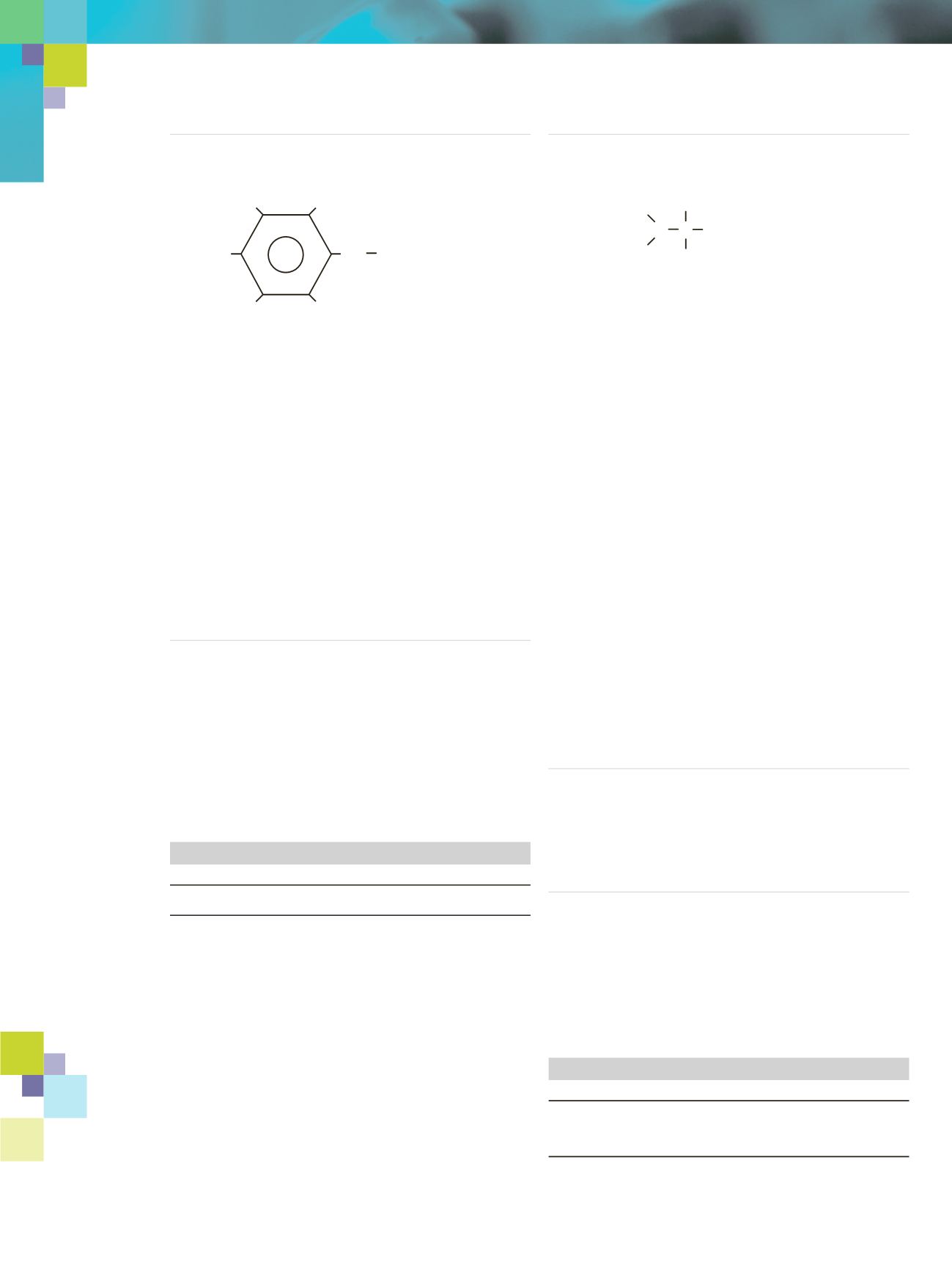
Pentafluorobenzyl Bromide (PFBBr)
For the electron capture GC analysis of carboxyl acids,
phenols and sulfonamides.
20
PFBBr
MW 260.9
bp 174-175
˚
C
d
4
1.86
Br
CH
2
F
F
F
F
F
Pentafluorobenzylation by an “Extraction Alkylation”
technique has been described for the electron capture GC
analysis of carboxyl acids, phenols and sulfonamides. This
process uses tetrabutylammonium as a counter ion and
methylene chloride as a solvent. Reaction times are fast
(~20 minutes). Derivatives are highly EC-sensitive, making
them useful in low-level determinations of fatty acids.
Kawahara performed extensive work with this reagent,
using a potassium carbonate catalyst for the electron
capture analysis of mercaptans, phenols and organic acids
in surface water.
PFBBr has been applied in analyzing trace organics in
asphalts, as a “fingerprinting” technique for identifying
asphalt pollutants found in surface waters.
PROTOCOL
For pentafluorobenzylation of acids, phenols and sulfonamides.
1. Place 1 ml methylene chloride containing 0.2 mg sample in a 3 ml
Thermo Scientific Reacti-Vial Small Reaction Vial.
2. Add 1 ml aqueous 0.1 M TBA hydrogen sulfate and 0.2 M sodium
hydroxide solution.
3. Add 20 µl PFBBr.
4. Cap vial and shake for 20-30 minutes.
5. Inject a portion of the methylene chloride phase into the chromatograph
for FID analysis.
6. Evaporatemethylenechloridetodrynesswithnitrogen and redissolve with
benzene for ECD analysis.
Ordering Information
Product # Description
Pkg. Size
TS-58220
PFBBr
5 g
(Pentafluorobenzyl Bromide)
Methylate Reagent (DMFDMA)
For easy, effective preparation of methyl esters from fatty
acids and amino acids.
20
Methylate Reagent
MW 119.17
bp 102-104
˚
C
d
4
0.897
N C H
OCH
3
OCH
3
CH
3
CH
3
For preparing methyl esters for gas chromatography,
Thermo Scientific Methylate Reagent offers significant
advantages including:
• Speed
– the reaction is complete upon dissolution
• Quantitation
– quantitative yields are obtained when
reagent and sample are injected – without prior mixing
• Your choice of formulation
– our Methylate Reagent is a
convenient, ready-to-use reagent that contains 2 mEq/ml
in pyridine.
Our Methylate Reagent is stable at room temperature
and is packed in convenient, ready-to-use Hypo-Vial
Sample Storage Vials. No water washing, extraction or
concentration of the derivatives are required. Plus, no water
is formed in the reaction.
Reactions with the Methylate Reagent usually are complete
upon dissolution, except for long chain solid acids. In these
applications, it is necessary to use Methylate Reagent
with additional solvent and mild heating. Suitable solvents
include pyridine, benzene, methanol, chloroform, methylene
chloride, THF and DMF.
Thenot,
et al.
have demonstrated analytical applications that
use of the Methylate Reagent for analyzing fatty acids
1
and
amino acids.
2
PROTOCOL 1
Methods of alkylation using DMF-Dialkyl Acetal Reagents.
1. Combine 50 mg fatty acid and 1 ml Methylate Reagent in a 3 ml Thermo
Scientific Reacti-Vial Small Reaction Vial.
2. Cap vial and heat at 60°C for 10-15 minutes or until dissolution is complete.
3. Analyze by gas chromatography.
PROTOCOL 2
For preparing
N
-dimethylaminomethylene (DMAM) methyl esters of
amino acids.
1. Combine amino acid with 1:1 ratio of Methylate Reagent to acetonitrile
in a Thermo Scientific Reacti-Vial Small Reaction Vial.
2. Cap vial and heat at 100°C for 20 minutes or until dissolution is complete.
3. Analyze by gas chromatography.
NOTE:
Aspartic acid requires a longer time for complete dissolution. Hydroxyl
groups on hydroxyl-substituted amino acids do not react under the above
conditions.
Ordering Information
Product # Description
Pkg. Size
TS-49350
Methylate Reagent
(2 mEq/mI
25 ml
in pyridine)
Hypo-Vial Sample
(
N
,
N
-Dimethylformamide
Storage Vial
dimethyl acetal)
Thermo Scientific Alkylation Reagents


