16
For more information, or to download product instructions,
Thermo Scientific Acylation Reagents
MBTFA
For trifluoroacylating primary and secondary amines,
hydroxyl and thiol groups, and carbohydrates.
20
MBTFA
MW 223.08
bp 123-124
˚
C
d
4
1.55
C CF
3
N C CF
3
O
O
CH
3
Thermo Scientific MBTFA is ideal for trifluoroacylating
primary and secondary amines, hydroxyl and thiol groups,
or carbohydrates under nonacidic conditions. In addition,
MBTFA is used to selectively acylate amines in the
presence of hydroxyl and carboxyl functions. The reaction
byproduct,
N
-methyltrifluoroacetamide, is volatile. MBTFA
also produces very volatile derivatives of carbohydrates.
Highlights:
• Trifluoroacetylates primary and secondary amines, as
well as hydroxyl and thiol groups under mild nonacidic
conditions
• Principle byproduct from the derivatization reaction is
N-methyltrifluoroacetamide, which is stable, volatile and
does not present problems in subsequent GC
• Produces very volatile derivatives of carbohydrates and
can be used to selectively acylate amines in the presence
of hydroxyl and carboxyl groups that have been protected
by silylation
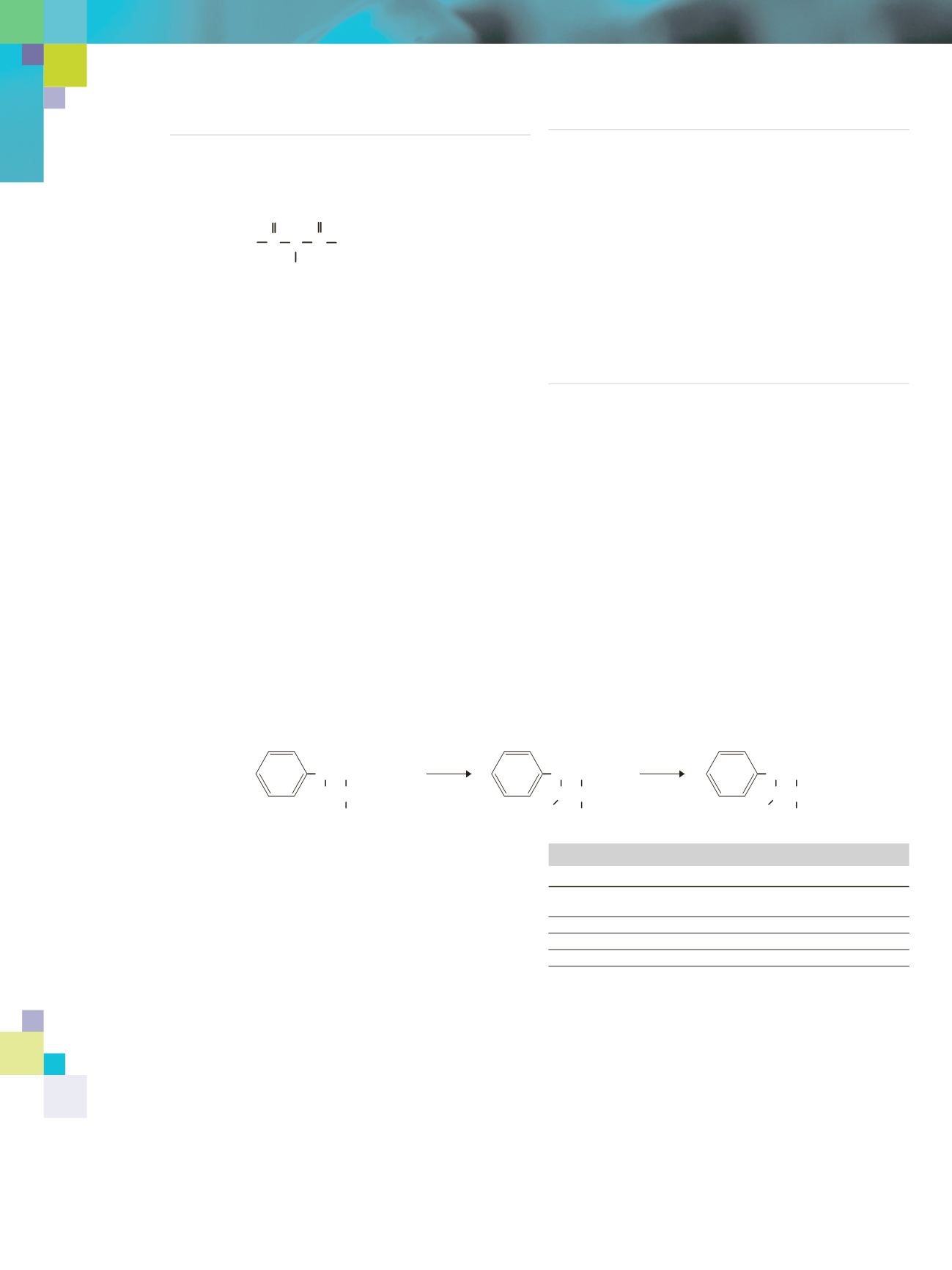
Selective acylation of amine groups in the presence of hydroxyl
and carboxyl groups
is possible if these groups are first protected by
silylation. The multifunctional compound first is silylated with MSTFA
(
N
-Methyl-
N
[TMS] trifluoroacetamide), which silylates all of the
functional groups (TMS is trimethylsilyl). The compound then is further
reacted with MBTFA, exchanging the TMS-group on the amino function
with a trifluoroacetyl group. Similar results are obtained with amino
acids that yield
N
-Trifluoroacetyl-O-TMS-esters.
PROTOCOL 1
For trifluoroacylating primary and secondary amines, and hydroxyl and
thiol groups.
1. Combine 1-10 mg sample and 0.1-0.2 ml MBTFA. If sample is not easily
solubilized, add 0.5-1.0 ml pyridine, DMF, THF or acetonitrile. (MBTFA can
be pre-mixed with solvent in a 1:4 ratio. Add 1 ml pre-mixed solution to
1-10 mg compound.)
2. Cap vial and heat at 60-100°C for 10-30 minutes (longer for hindered
compounds).
3. Cool and analyze by gas chromatography.
NOTE:
MBTFA reacts with amines at room temperature to yield quantitative
results in approximately 30 minutes. Hydroxyl groups are slower to react.
As a general procedure, reaction mixtures should be heated for 10-30
minutes at 60-100°C. In the case of hindered compounds, further heating
may be necessary.
PROTOCOL 2
For trifluoroacylating sugars.
Producing TFA derivatives of sugars using standard fluorinated
anhydride and fluorinated acylimidazole procedures has yielded
multiple or unstable derivatives. MBTFA produces the corresponding
trifluoroacetyl derivatives of the mono-, di-, tri- and tetrasaccharides.
These derivatives, when subjected to gas chromatography, produce
quantitative results and yielded an unexpectedly high degree of
volatility.
The high volatility of the corresponding TFA derivatives yields shorter
retention times at lower temperatures than other commonly used
silylation methods. The result is that polar columns with lower maximum
temperature limits can be used to provide faster separations under less
stringent chromatographic conditions. Mixtures of carbohydrates
containing mono- through tetrasaccharides can be analyzed in a single
run in as little as 15 minutes.
1. Combine 5-10 mg dry sugar and 0.5 ml MBTFA in a 5 ml Thermo Scientific
Reacti-Vial Small Reaction Vial.
2. Add 0.5 ml pyridine.
3. Cap vial and heat at 65°C for 1 hour with occasional shaking.
4. Analyze 1 µl by gas chromatography.
NOTE:
Reactions are complete upon dissolution.
Ordering Information
Product # Description
Pkg. Size
TS-49700
MBTFA
10 x 1 ml ampules
[N-Methyl-bis(trifluoroacetamide)]
TS-49701
MBTFA
5 g bottle
TS-49703
MBTFA
25 ml
✖
TS-49704
MBTFA
100 ml
✖
Additional hazardous handling charge.
MSTFA
CH–CH–CH
3
OH N–CH
3
•
HCl
H
MSTFA
CH–CH–CH
3
O N–CH
3
TMS
TMS
CH–CH–CH
3
O N–CH
3
COCF
3
TMS


