Thermo Scientific
| Reagents, Solvents and Accessories
41
Thermo Scientific High-Purity Pre-Column Derivatization Reagents
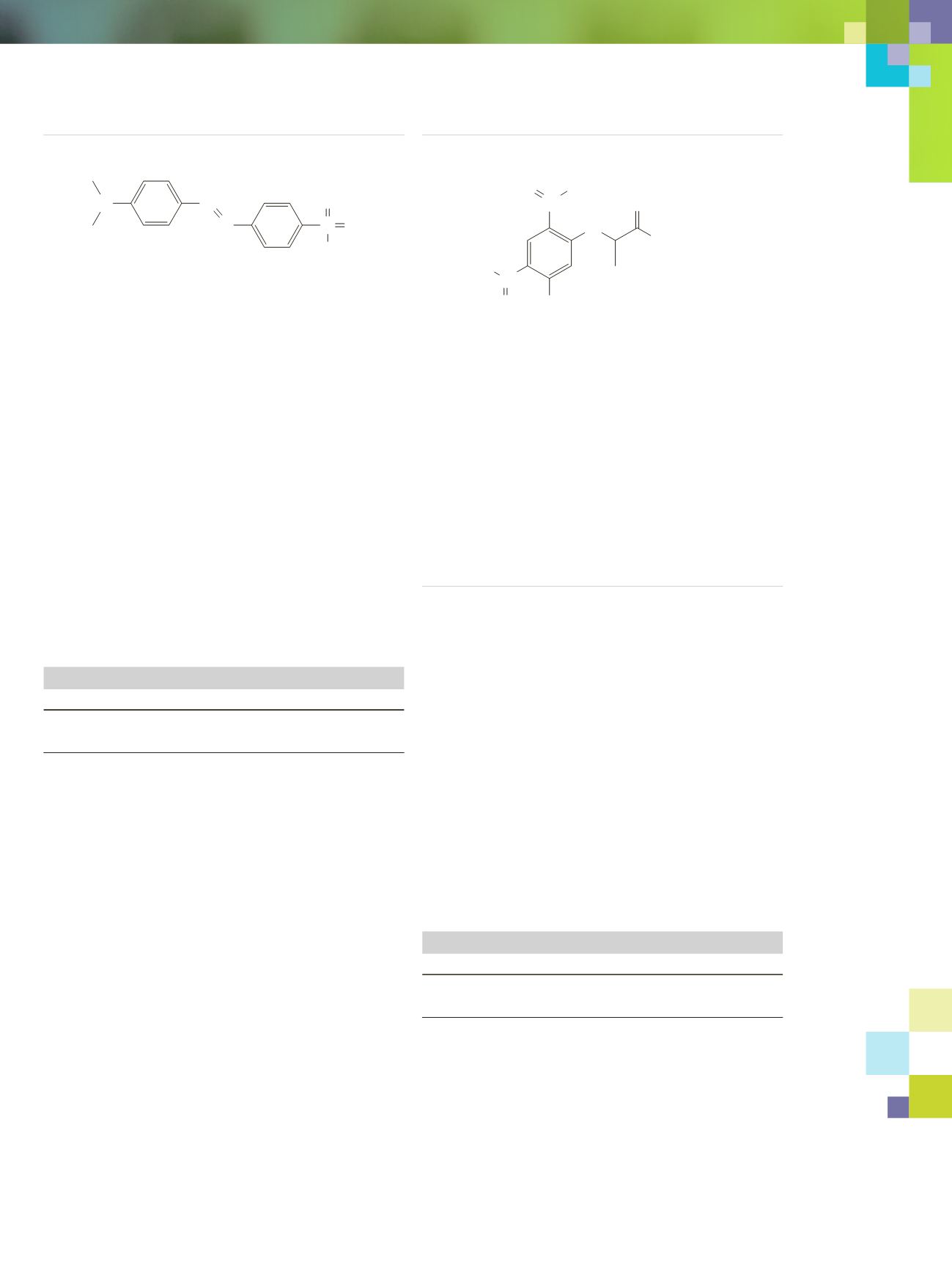
Dabsyl Chloride
It’s recrystallized twice for twice the quality!
N
N
N
S
Cl
O
O
Dabsyl Chloride
MW 323.80
Thermo Scientific Dabsyl Chloride is ideal for the
pre-column derivatization and detection of amino acids
in visible light down to sub-picomolar levels, followed by
reverse-phase HPLC.
Highlights:
• Analysis of 10-30 ng of protein hydrolyzates
1,2
• Analysis of peptides and determination of
C-terminal sequence of polypeptides
1
• Analysis of phospho-amino and amino acid amides
3
• Analysis of amino acid neurotransmitters in mouse brain
4
• Optimal reaction conditions
5
References
1. Chang, J.Y.,
et al.
(1981).
Biochem. J.
199
, 547-555.
2. Chang, J.Y.,
et al.
(1982).
Biochem. J.
199
,
803-806
3. Chang, J.Y. (1984).
J. Chromatogr.
295
, 193-200.
4. Chang, J.Y.,
et al.
(1981).
FEBS Lett.
132
, 117-120.
5. Vendrell, J.,
et al.
(1986).
J. Chromatogr.
358
, 401-413.
6. Lin, J.K.,
et al.
(1980).
Clin. Chem.
26
, 579-583
7. Chang, J.Y.,
et al.
(1983).
Methods. Enzymol.
92
, 41-48.
8. Stocchi, V.,
et al.
(1985).
J. Chromatogr.
349
, 77-82.
Ordering Information
Product # Description
Pkg. Size
✖
TS-21720
Dabsyl Chloride
500 mg
(4-Dimethylaminoazobenzene
-4-Sulfonyl Chloride)
✖
Additional hazardous handling charge.
FDAA, Marfey’s Reagent
Derivatizes optical isomers of amino acids in just 90
minutes.
H
N
NH
2
O
F
N
+
–
O
O
N
+
O
–
O
FDAA
(Marfey’s Reagent)
MW 272.19
Thermo Scientific FDAA, (1-fluoro-2,4-dinitrophenyl-5-L-
alanine amide), offers complete derivatization of amino acid
isomers in 90 minutes. Derivatized amino acids then are
separated and quantitated by reverse-phase HPLC.
The nature of the reagent and the resultant reaction
products with D-diastereomers suggest that strong
intramolecular hydrogen bonding causes these derivatives
to elute much later than their L-diastereomer counterparts.
Derivatives have an absorption coefficient of approximately
3 x 10
4
. They can be detected by UV at 340 nm with picomole
sensitivity. Complete instructions are included with each
order.
TO PREPARE FDAA DERIVATIVES:
1. Place 100 µl (5 µmoles) sample in a 1.0 ml Thermo Scientific Reacti-Vial
Small Reaction Vial.
2. Add 200 µl of 1% (w/v) solution of FDAA in acetone. Add 40 µl of 1.0 M
sodium bicarbonate (µmoles FDAA: µmoles amino acid should be 1.5:1.0.)
3. Heat at 40°C for 1 hour. Remove and cool.
4. Add 20 µl 2 M HCI. Allow sample to degas.
5. Analyze.
Conditions: Hypersil GOLD 100mm x 4.6mm (part number 25005-104630)
UV at 340 nm
A: 0.05 M TEA phosphate pH 3.0
B: CH
3
CN
Linear Gradient, 10% B to 40% B in 45 minutes
Flow: 2.0 mI/min. at 25°C
References
1. Marfey, P.,
et al.
(1984).
Carlsberg Res. Comm
.
49
, 585-590.
2. Marfey, P. (1984).
Carlsberg Res. Comm.
49
, 591-596.
3. Szókán, G.,
et al.
(1988). Applications of Marfey’s Reagent in racemization stud-
ies of amino acids and peptides.
J. Chromatogr.
444
,115-122.
4. Aberhart, D.J.,
et al.
(1985). Separation by high-performance liquid chromatog-
raphy of (3R)- and (3S)-B-Leucine as diastereomeric derivatives.
1
151
, 88-91.
5. Martinez del Pozo,
et al.
(1989). Stereospecificity of reactions catalyzed by bac-
terial D-amino acid transaminase.
J. Biol. Chem.
264(30)
17784-17789.
Ordering Information
Product # Description
Pkg. Size
TS-48895
FDAA Marfey’s Reagent
50 mg
(1-fluoro-2, 4-dinitrophenyl-5-L
-alanine amide)


