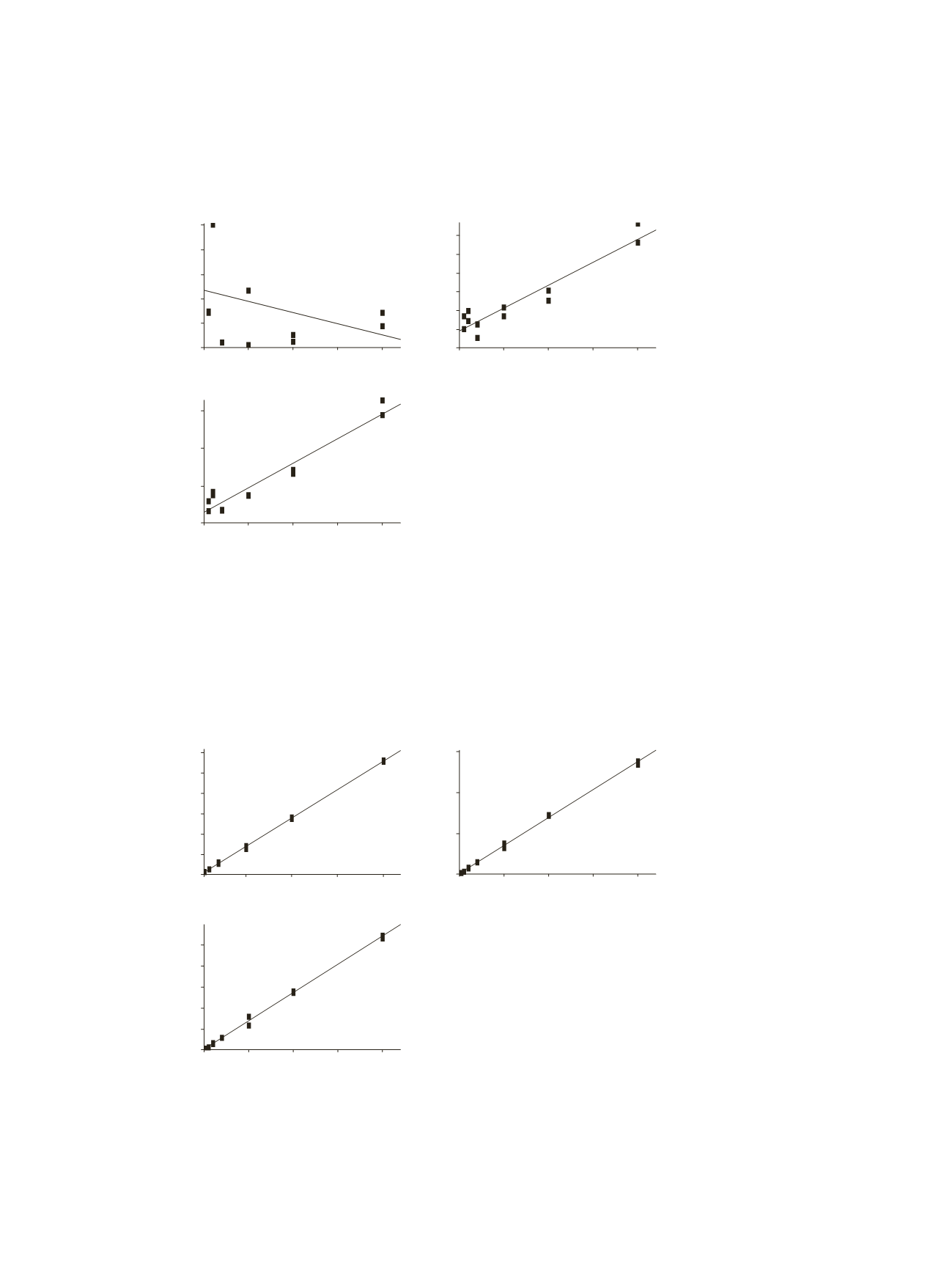
4
Figure 1: Calibration curves for bifenthrin and permethrin isomers (50–2000 ng/mL) using non-silanized amber
glass vial
To determine if the glass vial was responsible for adsorption of pyrethroids, it was decided to
substitute the non-silanized amber vials with an alternative plastic vial. The results obtained
showed improved recovery and linearity of sample response (Figure 2, Table 2). However, the
plastic vials, composed of polypropylene, would also be likely to introduce leachable organic
species when exposed to the organic solvent for any extended period of time. The contact time
between the solvent and plastic vials had to be kept to a minimum to avoid introduction of these
polypropylene extractables into the mass spectrometer.
Figure 2: Calibration curves for bifenthrin and permethrin isomers (50–2000 ng/mL) using a plastic vial
To determine if the type of glass could have an effect on adsorption of pyrethroids, a high purity
clear neutral borosilicate glass vial was evaluated. Thermo Scientific National 9 mm Target DP
Total Recovery Vials, 33 expansion borosilicate clear (Type 1, Class A) were used. This gave
improved linearity and extraction recoveries for all pyrethroids (Figure 3, Table 2). In this case, the
contact time was not found to be a limiting factor, and the pyrethroid samples could be safely
stored in the vial, if shielded from direct sunlight.
Results
Due to the possible breakdown of pyrethroids if exposed to light during sample preparation,
standard solutions were initially prepared in amber glass vials. Investigation showed that the choice
of a non-silanized 51A amber (type 1, class B) glass autosampler vial was impacting the recovery of
the compounds from the vials. The results showed that the overall recovery of the pyrethroids was
poor and the calibration of both bifenthrin and permethrin showed non-linear response (Figure 1).
The higher levels of iron oxide present in the amber vials used as a coloring agent leaches out when
in contact with water. The glass surface then becomes more active and interacts with pyrethroids.
Area Ratio
500
0
0.0000
0.0001
0.0002
0.0003
0.0004
0.0005
1000
1500
2000
ng/mL
Area Ratio
500
0
0.0000
0.0001
0.0002
0.0003
0.0004
0.0005
0.0006
1000
1500
2000
ng/mL
Area Ratio
500
0
0.0000
0.0005
0.0010
0.0015
1000
1500
2000
ng/mL
Area Ratio
500
0
0
1
2
3
4
5
6
1000
1500
2000
ng/mL
Area Ratio
500
0
0.0
0.5
1.0
1.5
1000
1500
2000
ng/mL
Area Ratio
500
0
0.0
0.5
1.0
1.5
2.0
2.5
1000
1500
2000
ng/mL
Bifenthrin
Permethrin Isomer A
Permethrin Isomer B
Bifenthrin
Permethrin Isomer A
Permethrin Isomer B


