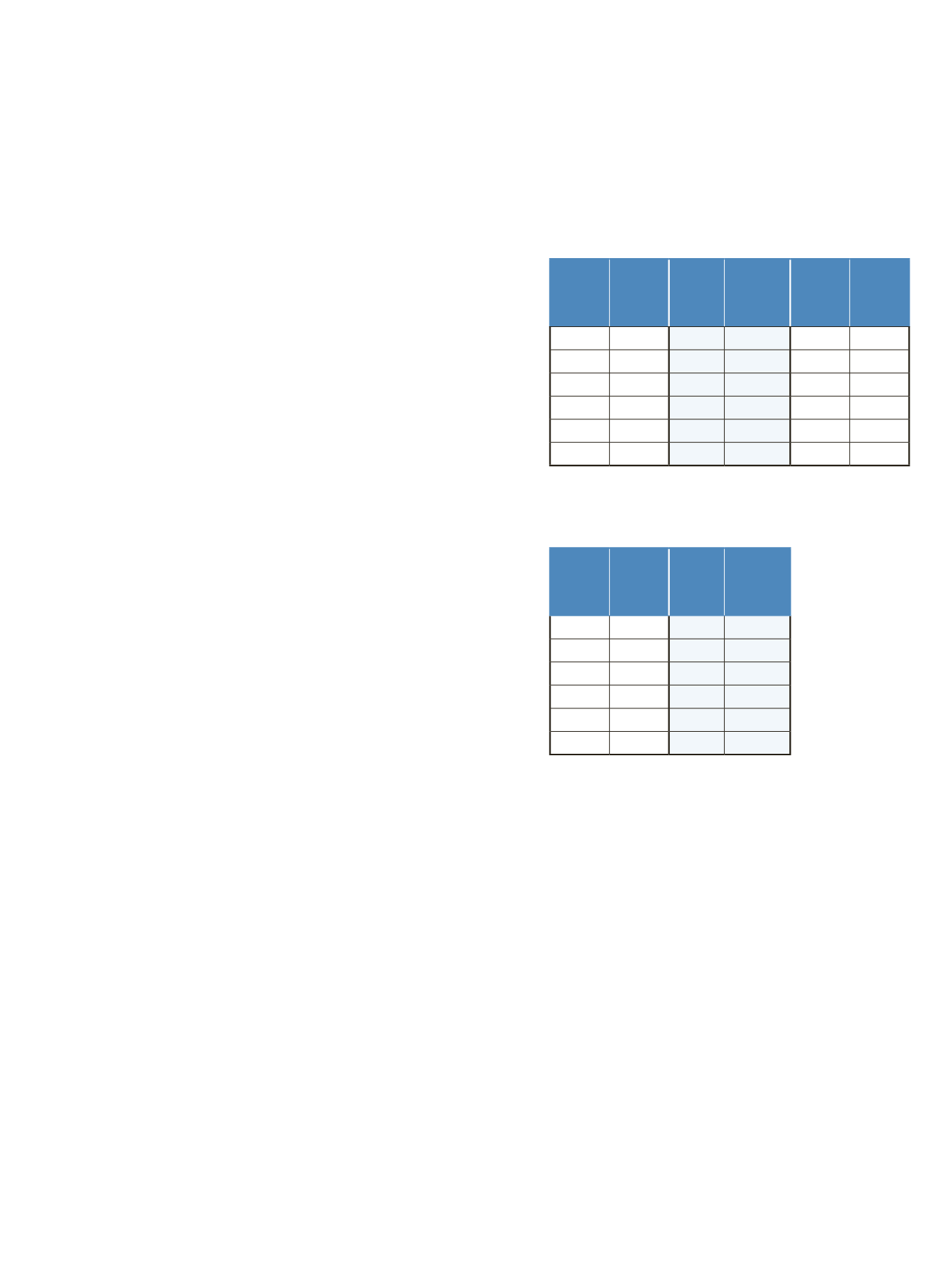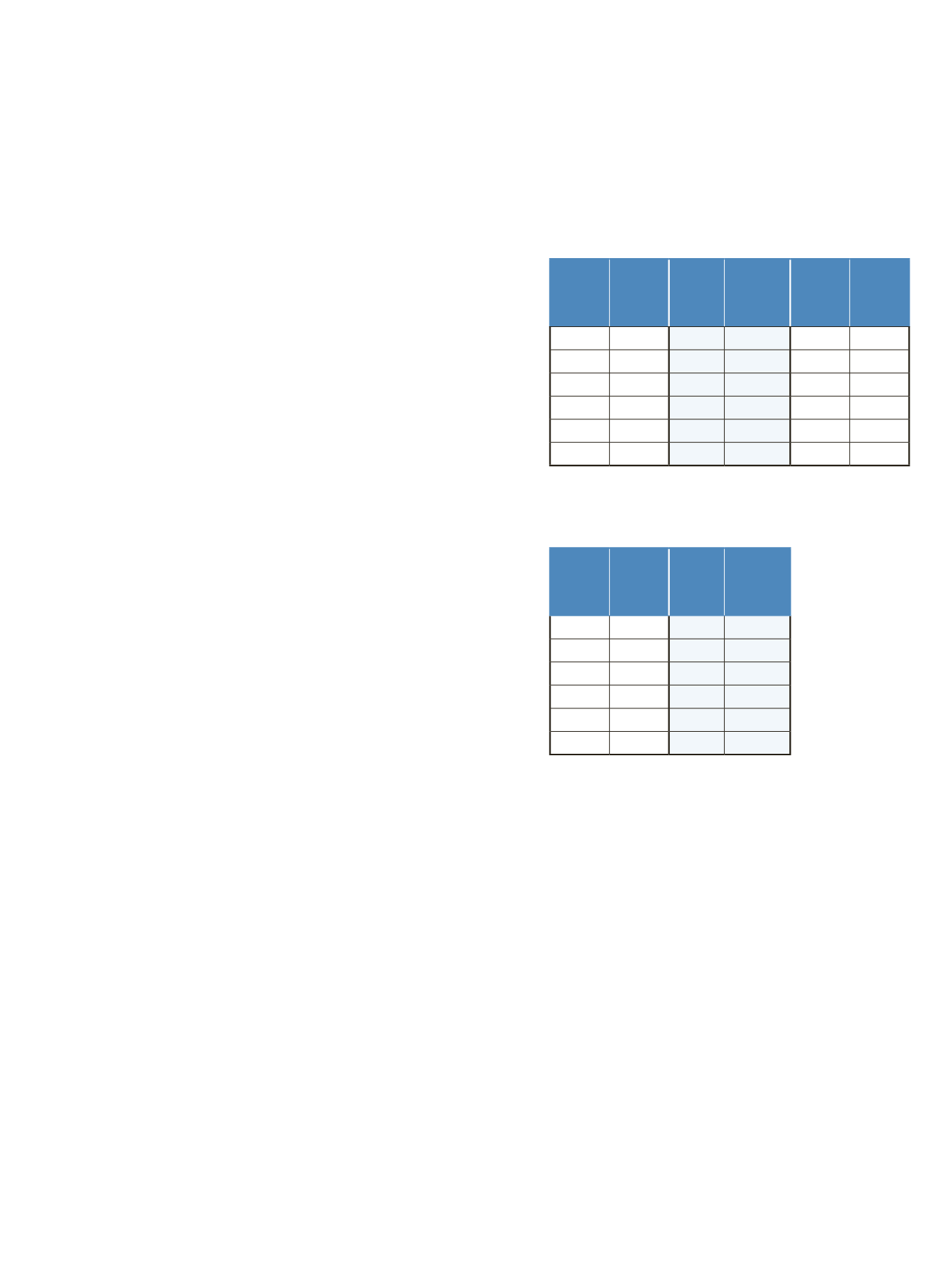
2
Experimental
Sample Preparation
The commercially available monoclonal antibody rituximab
was used in all experiments. Rituximab is a sterile, clear,
colorless, preservative-free, concentrated solution for
intravenous infusion. It was supplied at a concentration of
10 mg/mL, formulated in 7.35 mg/mL sodium citrate buffer
containing 0.7 mg/mL polysorbate 80, 9.0 mg/mL sodium
chloride, and sterile water, and ready for injection. The pH
was adjusted to 6.5 with sodium hydroxide or hydrochloric
acid.
Prior to LC/MS analysis, rituximab was dialyzed due to
polysorbate 80 in the sample. The dialysis was performed
with a Thermo Scientific
™
Slide-A-Lyzer
™
dialysis cassette
with a molecular weight cut off (MWCO) of 3.5 kDa.
A 1 mL sample of rituximab was dialyzed for 48 h against
2 L of 20% aqueous acetonitrile (ACN) at 4 °C.
For analysis of the light and heavy chains of rituximab,
disulfide bonds were reduced by incubation for 30 min at
60 °C with 5 mM tris(2 carboxyethyl)phosphine (TCEP).
For the bottom-up analysis of digested mAb, the sample was
alkylated with 20 mM iodoacetamide (IAA) for
30 min at room temperature in the dark after the reduction
step. The sample was purified with Thermo Scientific
™
Pierce
™
C18 tips dried in a Thermo Scientific
™
SpeedVac
™
concentrator and dissolved in 0.5 M triethylammonium
bicarbonate buffer (TEAB). Sequencing grade modified
trypsin (Promega) was added twice in a total ratio of
1:15 (w/w) at 0 h and 1.5 h and digestion was allowed
to proceed for 2.5 h at 37 °C. The digest was stopped by
addition of triflouroacetic acid (TFA) to approximately
pH 3.
All samples were supplied in autosampler vials containing
glass inserts (micro-inserts 0.1 ml, clear glass, VWR).
Liquid Chromatography
A monolithic 160 x 0.20 mm i.d. poly(styrene-
divinylbenzene) copolymer (PS-DVB) capillary column,
prepared according to a previously published protocol
1
, and
a Thermo Scientific
™
PepSwift
™
monolithic 250 x 0.20 mm
i.d PS-DVB capillary column were used. Protein separations
were performed with a Thermo Scientific
™
Dionex
™
UltiMate
™
3000 RSLCnano system that included a detector
equipped with a 3 nL z-shaped capillary detection cell.
Separations were accomplished at 55 °C with a gradient
of 20–60% acetonitrile (ACN) in 0.050% aqueous
triflouroacetic acid (TFA) in 10 min at a flow rate of
1 µL/min. For the proteolytic digest with trypsin, the
gradient was adapted to run at 0–50% B in 30 min.
For the reduced antibody samples, a gradient from
35–45% B in 15 min was selected.
Protein separation in a higher scale was performed using a
Thermo Scientific
™
ProSwift
™
RP-10R monolithic 50 mm x
1.0 mm i.d. column with an UltiMate 3000 RSLCnano
system that included a 45 nL detection cell. The column was
run with a flow rate of 60 µL/min and a column temperature
set to 55 °C. The gradient used was 26–80% B in 20 min.
For the reduced antibody, a gradient of 26–56% B in 20 min
was chosen to separate the heavy and the light chain.
The recorded back pressure of the monolithic columns for
the gradients described above was in the range of 190 to
260 bar for the PepSwift 250 mm x 0.2 mm i.d. column and
120 to 180 bar for the ProSwift RP-10R 50 mm x 1 mm i.d.
column.
For all experiments, the solvents used were water with
0.05% TFA (A) and acetonitrile with 0.05% TFA (B).
The LC gradients are described in Tables 1 and 2.
Table 1. LC gradients used for experiments with the PepSwift
250 mm x 0.2 mm i.d. column, at a flow rate of 1 μL/min
Mass Spectrometry
The Q Exactive benchtop Orbitrap mass spectrometer
was used for all experiments in this study. Experiments
using the ProSwift RP-10R 50 mm x 1 mm i.d. column
were performed using the Thermo Scientific
™
IonMax
™
source with the heated electrospray ionization (HESI)
sprayer, applying 4 kV spray voltage and sheath gas and
auxiliary gas flow rates of 15 and 5 units, respectively.
All other experiments were performed using the
Thermo Scientific
™
NanoFlex
™
ion source equipped with
15 cm PicoTip
®
emitter (New Objective, Woburn, USA;
20 µm i.d., 360 µm o.d., 10 µm tip), running with a flow
rate of 1 µL/min. A source voltage of 1.5 kV was applied.
Method details are provided in Table 3.
Table 2. LC gradient used for experiments with the
ProSwift RP-10R 50 mm x 1 mm i.d. column, at a flow rate
of 60 μL/min
Time
[min]
Intact
mAb
[%B]
Time
[min]
Reduced
mAb
[%B]
Time
[min]
mAb
Digest
[%B]
0.0
20
0.0
35
0.0
0
10.0
60
15.0
45
30.0
50
10.1
85
15.1
85
30.1
85
16.0
85
21.0
85
40.0
85
16.1
20
21.1
35
40.1
0
30.0
20
30.0
35
50.0
0
Time
[min]
Intact
mAb
[%B]
Time
[min]
Reduced
mAb
[%B]
0.0
26
0.0
26
15.0
80
15.0
56
20.0
80
15.1
80
20.1
26
20.0
80
30.0
26
20.1
26
30.0
26