
6
A Novel On-Line Sample Cleanup and Liquid Chromatography Platform for LC/MS Analysis in the Clinical Research Laboratory
Conclusion
Far too often LC/MS methods and instruments fall short of the rigorous performance
criteria Clinical Research Labs require for everyday testing. The complex nature of the
samples being injected on the system and the number of samples which need to be
processed tax the instrumentation and columns. The system suitability method we
developed proved a valuable whole-system testing procedure and demonstrated
consistent performance of the Prelude SPLC systems in three different locations. This
purpose-designed testing facilitates the implementation of rigorous evaluation
standards for LC-MS systems used for clinical research. Availability of a standard
system suitability test allows vendors and scientists to verify LC/MS system
performance under controlled conditions which are similar to actual operating
circumstances and has proven to be a valuable tool which is utilized from manufacture
to installation of Prelude SPLC Systems. System performance was also verified by
calibration and QC results for ISDs that matched expected values under typical
operating conditions at two different clinical research facilities.
Acknowledgements
The authors thank the following who hosted our testing program at their laboratories:
Dr. William Clarke & Autumn Breaud of Johns Hopkins Medical Center,
Dr. Mark Kellogg & Dr Roy Peake of Boston Children’s Hospital and
Dr. Sihe Wang & Jessica Gabler of the Cleveland Clinic.
MassCheck is a trade mark of ChromSystems Instruments & Chemicals GmbH, Grafelfing, Germany.
All other trademarks are the property of Thermo Fisher Scientific and its subsidiaries.
This information is not intended to encourage use of these products in any manners that might infringe the
intellectual property rights of others.
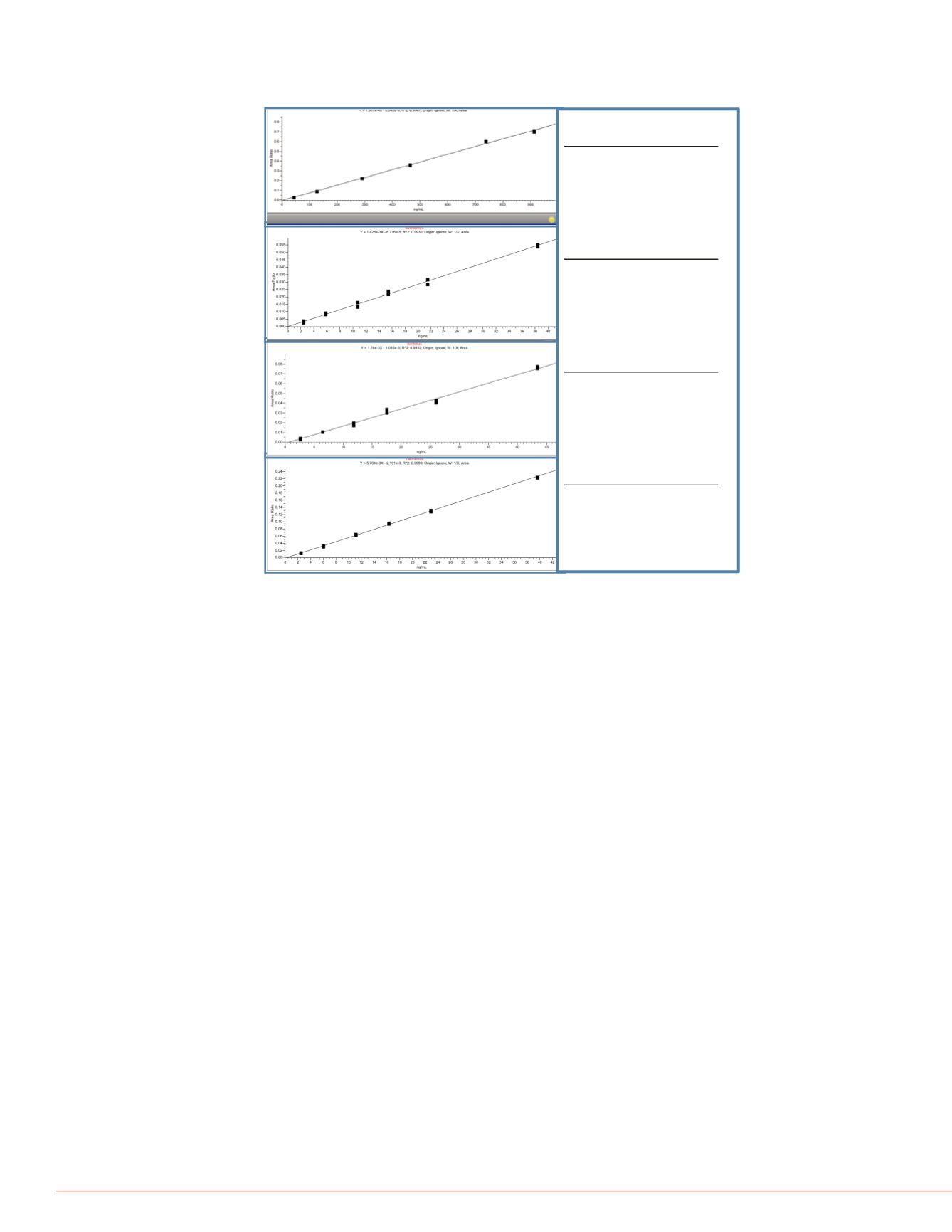
FIGURE 10. Quantitative Results Immunosuppressant Drugs
esearch Methods
Prelude SPLC system, popular LC-
dered. Methods for steroids, pain
nd 25-OH-Vitamin D2 and D3, were
within the experimental range, inter-
bility, solvent consumption as
arameters. Please see other posters
e 9 shows typical quantitative results
ibility for peak areas and retention
inearity.
ns
Cyclosporin A in whole-blood
preparation to detection of each
m. To evaluate the Prelude SPLC
romSystems® multilevel calibrators
essed using D
12
-Cyclosporin A
internal standard (IS) for Cyclosporin
icals, Canada) as the IS for
Ds of peak areas for the two IS
ive results, collected over a span of
- Cleveland, Baltimore and Boston,
in D2 and D3
Cyclosporin A QCs
Level Expected Average RSD
I
53 53 4.6%
II
276 260 3.5
III
514 515 2.1
IV
1111 1172 6.4
Everolimus QCs
Level Expected Average RSD
I
2.3 2.3 11.7%
II
4.4 4.4 11.0
III
8.5 8.8 8.4
IV
28.8 28.6 6.1
Sirolimus QCs
Level Expected Average RSD
I
2.9 2.9 8.5%
II
10.1 10.0 4.6
III
20.4 20.6 5.2
IV
38.5 38.6 6.2
Tacrolimus QCs
Level Expected Average RSD
I
2.6 2.8 5.3%
II
7.3 7.1 6.1
III
16.7 16.4 4.1
IV
34.2 33.8 4.1
n=15 from 3 systems within 30 days



















