
4
A Novel On-Line Sample Cleanup and Liquid Chromatography Platform for LC/MS Analysis in the Clinical Research Laboratory
RE 3. Peak Area %RSDs
sma spiked with our test mix was
trifuged. To test the reproducibility
ran a batch of 500 injections of the
e peak retention times and areas for
n Figure 2. Without the benefit of
k area RSDs were below 9%
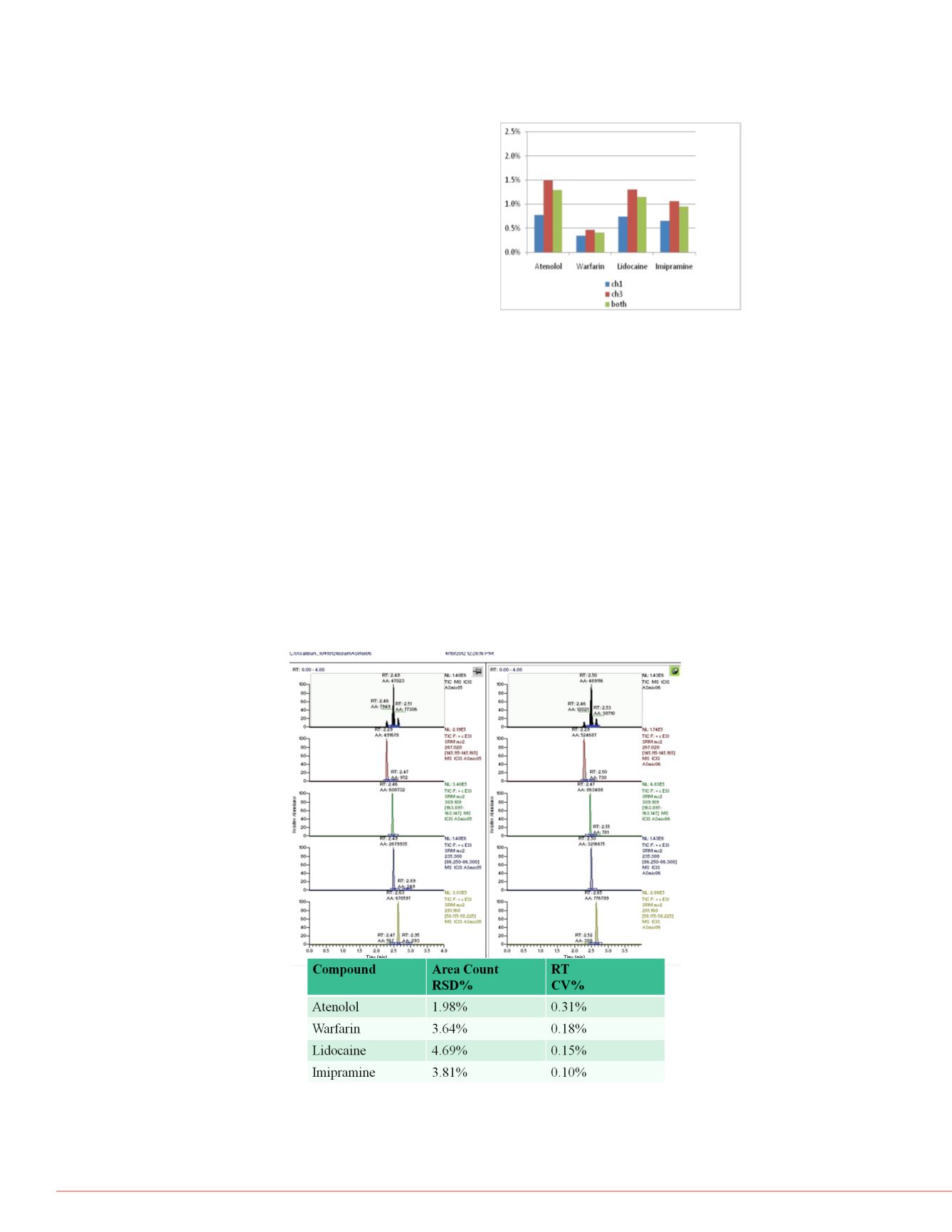
Inter-System Testing was Acceptable
While performance and ruggedness of any
single LC-MS system is essential, inter-
system performance is equally important.
The typical workflow from Development to
Production of a new method relies on inter-
system ruggedness and reproducibility. For
this reason, data from three prototype
SPLC systems were gathered over the
course of 5 months of testing and retention
time performance across the three systems
were analyzed. Reproducibility of retention
times for each of the four test compounds
generated from both channels of the three
systems is summarized in Figure 7. The
percent coefficient of variation (%CV)
values were calculated from A random
selection of 9 data points for each
compound.
A Purpose-Built Method clearly showed System Performance
Knowing that a rigorous LC-MS method with stringent data criteria would be the best
test of the Prelude SPLC system, a method that tests for common problems with
chromatographic separations was devised. The Suitability test has four compounds
the first, Atenolol, the earliest eluter, is used to help elucidate problems that might exist
with the refocusing of analytes on the analytical column. Retention time and peak
shape differences in Warfarin and Lidocaine peaks will help detect any problems that
may exist with gradient formation and/or column deterioration
,
as their RT shifts with
compositional mobile phase differences. Imipramine is highly susceptible to
degradation in peak shape if the mobile phases are not fresh or made precisely as
prescribed by the method. In concert, the test mix serves as powerful diagnostic tool.
The installation protocol for Prelude SPLC systems requires all four compounds to
pass 20 injections (10 per channel) with RSD or CV of 10% or less with no internal
standard correction for retention times and peak areas. Figure 8 shows typical
performance.
unds over 34 hrs of run time
rs & Peak Overlay at injections
1,100, 200, 3 and 500
FIGURE 8. System Suitability run of both channels of the Prelude SPLC System
ible
s to the removal of matrix effect and
ions and its impact on the aging of
nt. For that reason retention time
were evaluated for the same data
, pressure traces and peak shapes
on 34-hour batch.
System was Suitable for well-
In order to asses the scope of a
MS methods used in clinical res
management drugs, immunosu
developed and evaluated. We
and intra-day reproducibility, lo
compared to other platforms, a
for more details on some of the
for the Vitamin D compounds -
times while achieving the desir
Even for Immunosuppressan
Measuring Everolimus, Sirolimu
samples presents many challen
analyte and internal standard b
system’s ability to handle such
and MassCheck® whole blood
(Alsachim, Illkirch-Graffenstade
A and Tacrolimus-
13
CD
2
(Toront
Everolimus, Sirolimus and Tacr
compounds were less than 12
30 days from three systems in
are shown in Figure 9.
Imipramine
Lidocaine
Warfarin
Atenolol
FIGURE 7. Retention Time %CVs from 3
different prototype systems.
FIGURE 9. Quantitative Resul



















