
6
An Improved Immunosuppressant Drug Research Method Based on a Novel SPLC-MS/MS System
Conclusion
Improved reliability and economy was achieved for ISD analysis for
research purposes by using a novel SPLC-MS/MS system and method.
Ion suppression of ISDs by co-eluting phospholipids was largely avoided
by using the short Accucore C8 HPLC column.
Using 1/x weighting, correlation coefficients (r2) > 0.995 were typical for:
Cyclosporin A, from 25 to 1250 ng/mL,
Everolimus, Sirolimus & Tacrolimus, from 2.5 to 50 ng/mL.
Carryover, measured by peak areas corresponding to the ISDs from blank
injections following the highest calibrators, was typically less than 0.1%.
Reproducible ISD QC results were obtained from three research test sites
evaluating this method with the PreludeSPLC-TSQ Vantage system.
A reduction in solvent waste of about 40% was achieved, comparable to
legacy TurboFlow methods for ISDs.
Acknowledgements
The authors thank the following who hosted our testing program at their
laboratories:
Dr. William Clarke & Autumn Breaud of Johns Hopkins Medical Center,
Dr. Mark Kellogg & Dr Roy Peake of Boston Children’s Hospital and
Dr. Sihe Wang & Jessica Gabler of the Cleveland Clinic.
ChromSystems 6PLUS1 and MassCheck are registered trademarks of Chromsystems Instruments &
Chemicals, GmbH. All other trademarks are the property of Thermo Fisher Scientific and its subsidiaries
This information is not intended to encourage use of these products in any manners that might
infringe the intellectual property rights of others.
For Research Use Only. Not for use in diagnostic procedures.
Cs
o Significant Carryover
onsistently showed linear responses
Sirolimus and Tacrolimus and
rin A. Weighting the data by 1/x
nd calculated concentrations in
d QCs
Across 3 Different Test Sites.
re reported from three different
y, Boston Children’s Hospital and
C) Reproducibility Results
ithin 30 days
Matching Results from Legacy Method
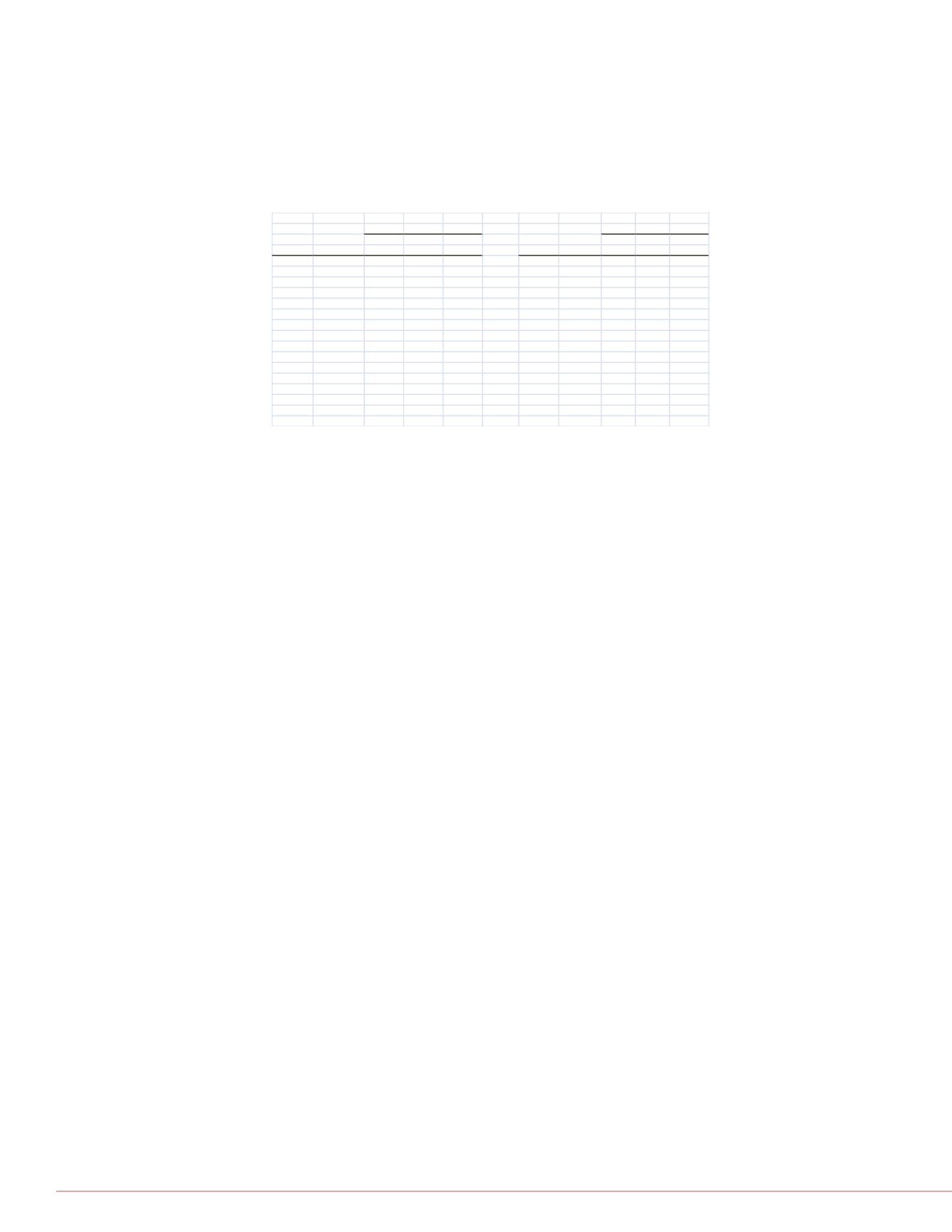
As shown in Table 2, the Prelude method produced results that agreed with
those produced by a legacy TurboFlow method for ISDs. Furthermore, the
Prelude results were reproduced remarkably well from sample preparations that
were almost 1 month old.
Repeated
Repeated
Ran on 1/9/2013 1/29/2013
Ran on 1/9/2013 1/29/2013
Test
Legacy Prelude Prelude
Test
Legacy Prelude Prelude
Sample
ISD
Method Method Method
Sample
ISD Method Method Method
8KLE Cyclosporin A:
86
105
103
120726-‐001 Everolimus:
3.5
3.0
4.5
8KBG Cyclosporin A:
186
201
203
120726-‐002 Everolimus:
2.0
1.7
1.8
8KOU Cyclosporin A:
84
99
93
120726-‐003 Everolimus:
2.0
1.8
2.0
8L20 Cyclosporin A:
80
81
75
120904-‐001 Everolimus:
4.0
4.3
3.9
8LB5 Cyclosporin A:
88
94
94
121227-‐001 Everolimus:
4.6
3.9
4.4
8JDF Cyclosporin A:
168
176
176
121227-‐002 Everolimus:
2.3
2.2
2.5
8I6C Cyclosporin A:
53
58
58
121227-‐003 Everolimus:
2.3
2.3
2.1
8KJNK
Sirolimus:
3.6
2.2
1.8
8LO5 Tacrolimus:
7.3
7.6
7.6
8KN6
Sirolimus:
3.0
1.2
2.0
8M3Y Tacrolimus:
2.6
3.2
2.9
8L5K
Sirolimus:
8.4
9.5
7.3
8M4D Tacrolimus:
12.5
11.1
12.5
8JB0
Sirolimus:
3.3
3.5
2.8
8M8F Tacrolimus:
2.3
2.8
2.8
8GOC
Sirolimus:
14.4
12.5
10.9
8MI1 Tacrolimus:
16.2
15.0
17.9
8I27
Sirolimus:
3.2
2.5
1.9
8MDV Tacrolimus:
8.9
8.8
9.6
86HF
Sirolimus:
5.7
5.5
4.2
8LRH Tacrolimus:
20.0
17.7
19.0
TABLE 2. Everolimus Calibrators and QCs
Everolimus
Expected Average RSD%
2.3
2.3 11.7
4.4
4.4 11.0
8.5
8.8 8.4
28.8
28.6 6.1
Tacrolimus
Expected Average RSD%
2.6
2.8 5.3
7.3
7.1 6.1
16.7
16.4 4.1
34.2
33.8 4.1



















