
2
An Improved Immunosuppressant Drug Research Method Based on a Novel SPLC-MS/MS System
Overview
Purpose:
Demonstrate robust and rugged method performance utilizing an
automated two-channel sample preparation-liquid chromatography (SPLC)
system that minimizes matrix interferences from whole blood when measuring
immunosuppressant drugs (ISDs) for research purposes by tandem mass
spectrometry (MS/MS) with electrospray ionization (ESI).
Methods:
A 5 minute method involved automated clean up of whole blood
preparations (cell rupture and protein precipitation by aqueous zinc sulfate and
methanol) using TurboFlow technology followed by high-resolution liquid
chromatography using a short Accucore C8, 2.6 µm HPLC column. Reversed-
phase extraction, elution and final separations were done in a way that avoided
the accumulation and co-elution of phospholipids, which would have suppressed
ionization of ISDs in ESI sources. Quantitation of four ISDs was achieved by
stable-isotope dilution using two internal standards (IS).
Results:
Performance specifications were consistently reproduced within systems
and across different laboratories as whole-blood levels were reliably measured:
between 2.5 and 50 ng/mL for Everolimus, Sirolimus and Tacrolimus; and
between 25 and 1,250 ng/mL for Cyclosporin A. A throughput of 21 samples per
hour was achieved when multiplexing across both channels, which generated only
165 mL of solvent waste. No significant carryover between samples was detected.
Introduction
Immunosuppressant drugs (ISDs) are often analyzed in whole-blood using LC-MS
with electrospray ionization, which is prone to interference by phospholipids.
Although stable isotopes for each ISD are available to compensate, minimizing
such interferences would improve data quality. The Thermo Scientific™ Prelude™
SPLC system—a novel dual-channel system that automates sample preparation
and liquid chromatography (SPLC), was interfaced to the ESI of a tandem mass
spectrometer (MS/MS) for the analysis of ISDs. The Prelude SPLC system
incorporated Thermo Scientific™ TurboFlow™ technology and high-efficiency LC
utilizing solid-core packing. Stable isotope derivatives D
12
-Cyclosporin-A and
Tacrolimus-
13
CD
2
were used as internal standards in the whole-blood sample
preparation procedure. The method was optimized to reliably minimize
interferences from phospholipids to improve data quality. The method was also
designed to minimize solvent waste.
Mass Spectrometry
The Thermo Scientific™ TSQ Van
heated electro-spray interface (
ammonium-adduct precursor io
Everolimus: 975.7 >
Tacrolimus: 821.5 >
Cyclosporin A: 1202.8
> 437.2
During method development, th
were tracked by adding the follo
Lyso-Phosph
Lyso-Phosph
Phosph
Data Analysis
Thermo Scientific™ TraceFinder
control, data acquisition and dat
above were used for quantitatio
Methods
Off-Line Sample Preparation
ChromSystems 6PLUS1® ISD m
blood controls as well as in-hou
sulfate solution and then with
Tacrolimus-
13
CD
2
(Toronto Res
(Alsachim, France). After centrif
autosampler vials.
On-Line Sample Preparation &
In each channel, 20 µL injection
Scientific™ TurboFlow™ Cyclone
mobile phase mixture of 7:3 wa
formate and 0.05% formic acid
extracted ISDs, which merged w
mixture, to transfer and focus t
HPLC column, which was mainta
were separated from matrix inte
ionization (HESI) source by a gra
focus method.
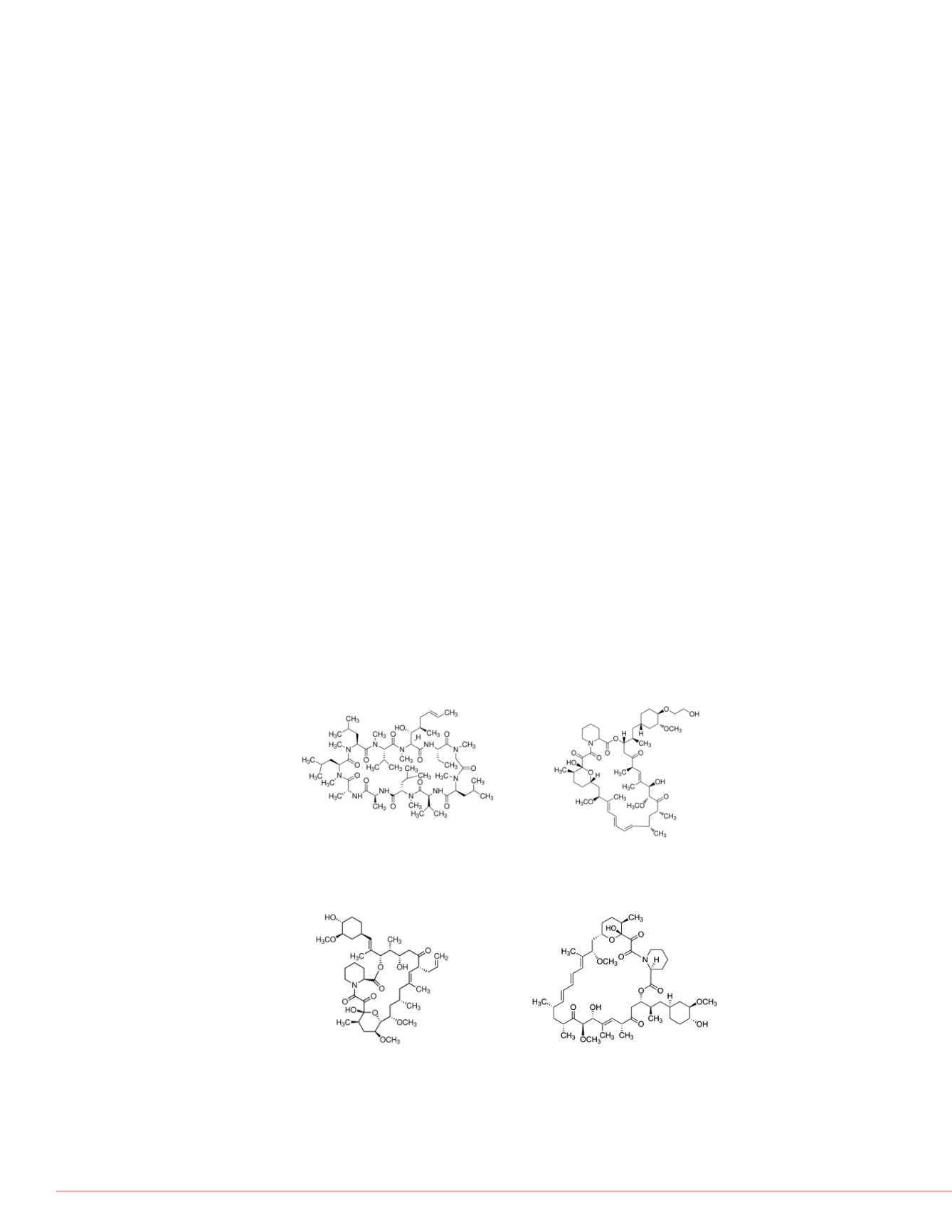
FIGURE 1. Immunosuppressant Drugs Analysed
Cyclosporin A
Everolimus
C
62
H
111
N
11
O
12
C
53
H
83
NO
14
MW: 1202.61 MW:
958.22
Tacrolimus (FK-506) Sirolimus
(Rapamycin)
C
44
H
69
NO
12
C
51
H
79
NO
13
MW: 822.03 MW: 914.17
FIGURE 2. Summary of SPLC F



















