
4
An Improved Immunosuppressant Drug Research Method Based on a Novel SPLC-MS/MS System
FIGURE 4. Optimized HPLC Conditions
Elution from Accucore C8, 2.6 µm, 3.0 x 30 mm column:
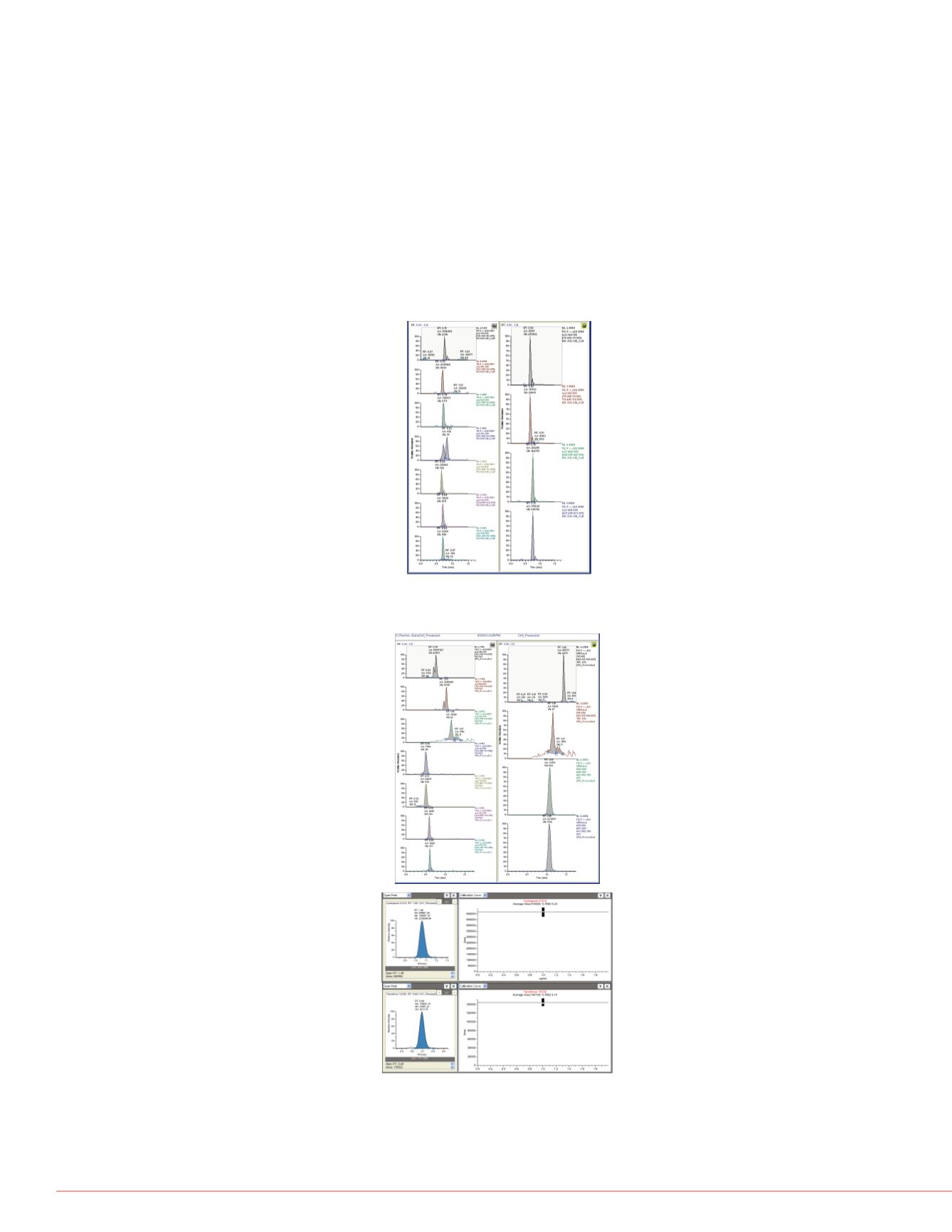
FIGURE 3. Non-Optimized HPLC Conditions
Elution from Accucore PFP, 2.6 µm, 3.0 x 50 mm column:
FIGURE 6. Everolimus Calib
e-stage quadrupole system with
used to measure the transitions from
ct ions:
Sirolimus: 931.6 > 864.6
Tacrolimus IS: 824.4 > 771.0
Cyclosporin A IS: 1214.9
phospholipids and dioctylphthalate
tions:
hthalate: 391 >149
e;16:0: 496 > 184
e;18:0: 524 > 184
e;38:6: 806 > 184
with Aria MX was used for instrument
g. The internal standards (IS) shown
isotope dilution technique.
Achieving Required Linear R
As shown in Figures 5 and 6, t
between 2.5 and 50 ng/mL for
between 25 and 1,250 ng/mL
minimized differences betwee
calibrators.
Results
Identifying the HPLC Column and Conditions that Minimize Interferences
Because ISDs are as hydrophobic as phospholipids and phthalates, all are
extracted and transferred to the HPLC column during the TurboFlow process.
Therefore, the HPLC conditions must be optimized to elute the ISDs to the
detector in a reasonable timeframe while avoiding co-elution of interferences
as well as buildup of interfering compounds in the HPLC column while
processing many samples. Figure 3 shows buildup and co-elution from non-
optimized conditions, which resulted in poor reproducibility (RSDs > 20%) of
peak areas for internal standards in sample batches. Figure 4 shows results
from optimized conditions, which resulted in improved IS peak area
reproducibility (RSDs < 10%).
ibrator set and MassCheck® whole-
ples were mixed with aqueous zinc
ntaining internal standards:
icals, Canada) and D
12
-Cyclosporin A
pernatants were harvested into glass
romatography (SPLC)
atants were extracted with a Thermo
Flow column (0.5 x 50mm) using a
ol containing 10 mM ammonium
in. A slow flow of methanol eluted
r flow of a 7:3 water: methanol
n Accucore C8, 2.6 um, 3.0 x 30 mm
°C by the built-in heater. The ISDs
nd eluted to the heated electrospray
creasing methanol. Figure 2 shows this
od.
Solvents:
A:
Water + 10mM NH
4
OOCH +
0.05% HOOCH
B:
Methanol + 10mM NH
4
OOCH
+ 0.05% HOOCH
C:
45% Acetonitrile + 45%
Isopropanol + 10% Acetone
Total solvent consumption is
3.37 mL A, 3.25 mL B, 1.5 mL C
for each injection.
Dioctylphthalate:
Lyso-Phosphotidylcholine;16:0:
Lyso-Phosphotidylcholine;18:0:
Phosphotidylcholine;38:6:
.Tacro:
Siro:
Evero:
:Tacro IS (824>771)
:Tacro IS (825>772)
:CycloA
CycloA IS
Lyso-Phosphotidylcholine;16:0:
Lyso-Phosphotidylcholine;18:0:
Phosphotidylcholine;38:6:
.Tacro:
Tacro IS:
Siro:
Evero:
:Dioctylphthalate
:Phosphotidylcholine;38:6
:CycloA
CycloA IS
FIGURE 7. Cyclosporin A Ca
Reproducible QC Results wer
As shown in Table 1, very simil
research test sites: Johns Hopk
The Cleveland Clinic.
TABLE 1. Commercial Qualit
n=15 fro
CyclosporinA
Level
Expected Average
I
53
53
II
276
260
III
514
515
IV
1111 1172
Sirolimus
Level
Expected Average
I
2.9
2.9
II
10.1
10.0
III
20.4
20.6
IV
38.5
38.6



















