
5
Thermo Scientific Poster Note
•
PN-60477-ASMS-EN-0614S
For forensic use only.
All trademarks are the property of Thermo
This information is not intended to encoura
intellectual property rights of others.
mpounds
TABLE 3. Transitions and MS parameters for all compounds
.5 4.0
4.14
0.5
1.0
1.5
2.0
2.5
3.0
Time (min)
0
20
40
60
80
100
0
20
40
60
80
100
0
20
40
60
80
100
Relative Abundance
0
20
40
60
80
100
1.65
1.47
1.79
1.65
1.46
1.79
2.38
odone
Hydromorphone-
3
b
-glucuronide
(1.65)
Codeine-6
b
-
glucuronide
Morphine-3
b
-
glucuronide (1.47)
Morphine-6
b
-
glucuronide (1.79)
Oxymorphon-3
b
-
glucuronide
1.46
Precision
(%RSD)
Intra-Assay
Inter-Assay
<14.3
<5.7
<14.1
<8.2
<8.8
<4.8
<10.3
<3.5
<14.1
<5.8
<9.6
<4.1
<11.1
<5.3
<13.6
<5.7
<13.5
<9.2
<14.1
<5.8
<11.6
<10.4
<7.4
<5.0
<9.7
<4.4
<8.5
<4.1
<14.4
<4.4
<7.9
<5.7
<14.9
<4.1
<10.8
<3.7
<6.1
<6.9
Analyte
Precursor
Ion (Q1)
Product
Ions (Q3)
CE
(V)
S-lens
(V)
Normorphine
272.0
165.0
59
95
209.0
40
95
Morphine-3
b
-glucuronide
462.1
286.1
52
148
185.2
58
139
Oxymorphone-3
b
-glucuronide
478.1
284.1
47
147
302.1
42
147
Hydromorphone-3
b
-glucuronide
462.1
185.2
58
139
286.1
52
148
Morphine-6
b
-glucuronide
462.1
286.1
52
148
185.2
58
139
Codeine-6
b
-glucuronide
476.2
300.2
31
114
215.2
39
114
6-Acetylmorphine
328.1
165.0
58
112
211.0
39
112
6-Acetylcodeine
342.1
225.1
27
109
165.1
47
109
Dihydromorphine
288.1
185.1
48
95
165.0
59
95
Morphine
286.1
165.1
64
90
185.0
44
119
Oxymorphone
302.0
227.0
40
116
199.1
55
116
Hydromorphone
286.1
185.0
44
119
165.1
64
90
Codeine
300.0
171.0
40
119
199.1
43
119
Dihydrocodeine
302.0
201.1
42
93
199.0
52
93
Norcodeine
286.1
165.1
64
90
181.6
49
90
Oxycodone
316.0
241.1
41
119
256.0
40
119
Noroxycodone
302.1
227.0
41
116
187.0
40
116
Norhydrocodone
286.1
199.0
39
119
241.1
35
119
Hydrocodone
300.0
171.1
40
119
181.1
51
94
Noroxycodone-d
3
305.1
190.1
25
116
Norhydrocodone-d
3
289.1
152.1
62
116
6-Acetylmorphine-d
6
334.1
165.1
38
116
Morphine-6
b
-glucuronide-d
3
465.1
289.1
32
140
Morphine-d
3
289.1
152.1
61
116
Dihydrocodeine-d
6
308.1
202.1
34
116
Codeine-d
6
306.1
165.1
43
116
Hydromorphone-d
6
292.1
185.1
32
116
Morphine-3
b
-glucuronide-d
3
465.1
289.1
31
140
Oxycodone-d
6
322.1
218.1
43
116
FIGURE 4. Molecular structu
hydromorphone (H)
Hydromorphone-3
b
D-glu
Conclusion
This quantitative method
and their metabolites wit
Verification of a forensic
Prelude SPLC system a
can analyze these proble
This forensic method is
less costly then those th
eliminated from the work
The Prelude SPLC syste
necessary chromatograp
of all compounds.
ms for all 19 compounds
Morphine-6
b
-
Glucuronide
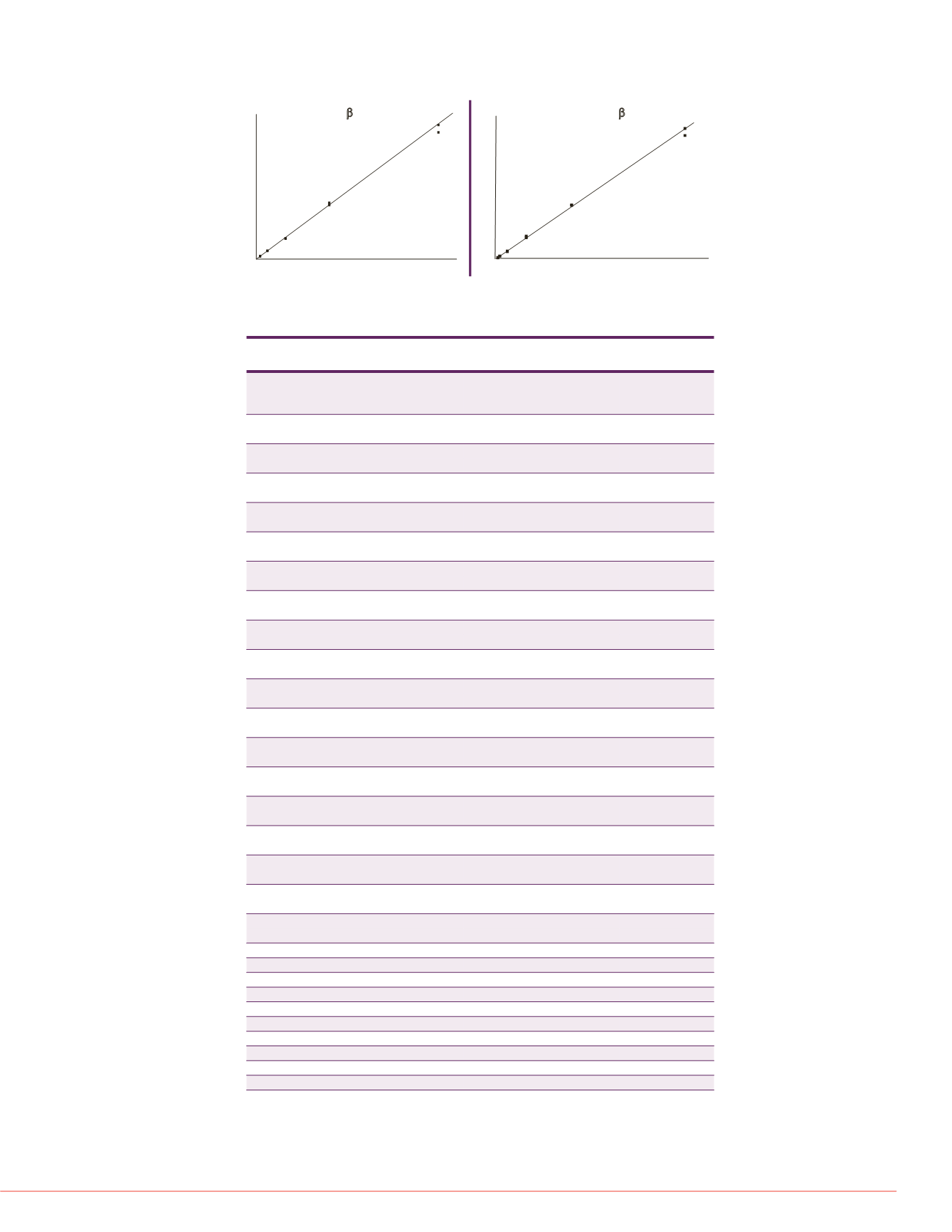
Area Ratio
Concentration (ng/mL)
Area Ratio
Oxymorphone-3
b
-
Glucuronide
Concentration (ng/mL)
FIGURE 3. Representative calibration curves for the intact glucuronides
Figure 4 displays molecular st
hydromorphone. The starting
molecule, which exists before
analyzes this structure as a w
Table 4 shows the results of a
nonhydrolyzed samples. Anal
results as does analyzing the
TABLE 4. Comparison of hydr
concentration from hydrolyze
Prepared concentration (ng/mL
Measured concentration prior t
Measured concentration after h
Expected % converted to pare
weight (285/461=62)
Actual % measured based on r
% Difference



















