
4
Results and Discussion
Chromatographic Method Development
Preliminary work indicated that 2-butoxyethanol needs to
be ionized in a very narrowly defined pH range in the
electrospray ionization source. Therefore, the pH was
kept constant throughout the run by adding the same
concentration of formic acid to both the aqueous and the
organic mobile phases. A solution of 0.1% formic acid in
water (pH 2.8) was used in combination with 0.1%
formic acid and 1% water in acetonitrile. This approach
provided acceptable peak shape and intensity for the
negative mode signals and allowed good ionization of
2-butoxyethanol (Figure 3).
Seawater Sample-Preparation Development
Signal suppression was observed for all analytes when
fortified, undiluted seawater was injected relative to
solutions of the same concentration in deionized water.
Two experiments were conducted to determine the
optimum dilution conditions that would provide adequate
signals for quantification. In a first experiment, acetonitrile
was compared to deionized water as a dilution solvent. A
fortified seawater sample was diluted from 100% to 50%,
with the dilution solvent being progressively changed
from deionized water to acetonitrile, while keeping the
dilution factor constant. As observed in Figure 4, the
DOSS peak area increased to a maximum as the percentage
of acetonitrile increased, indicating that acetonitrile was a
better dilution solvent than water.
Figure 4. Comparison between acetonitrile and deionized water
as solvents
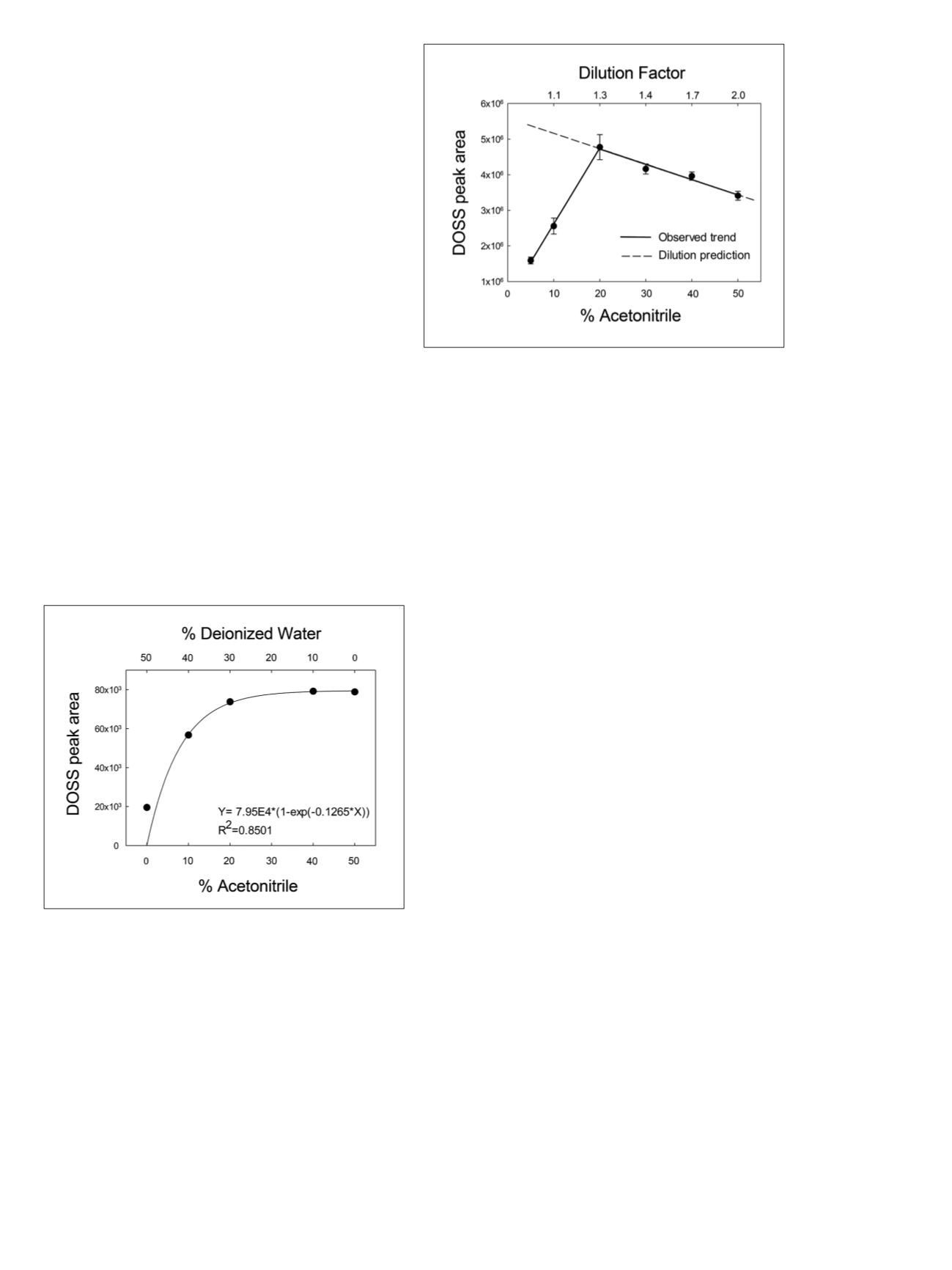
In a second experiment, the optimal seawater-to-acetonitrile
ratio was established by progressively diluting a fortified
seawater solution. Figure 5 shows that DOSS peak area
increases to a maximum between 20% and 30% v/v of
acetonitrile, before following the expected dilution trend.
Figure 5. Dilution experiment of a 10 µg/L DOSS-fortified seawater
sample
These results suggested that acetonitrile may reduce the
interaction between DOSS and the glass vial surface. To
investigate the storage effect of sample containers, 5 µg/L
DOSS-fortified seawater samples were stored in three
common types of sampling bottles (glass, PTFE, and PE)
at or below 4 °C. Subsamples were taken at 0, 1, 3, and
25 hours and analyzed. Based on the dilution experiment
results, a second set of fortified seawater samples were
stored in the same bottle types and acetonitrile was added
to 33% v/v (5:1 seawater/acetonitrile ratio). The results
are shown in Figure 6. In the absence of acetonitrile, the
recoveries of DOSS were severely reduced from the start
of the experiment in all three types of sampling bottles.
However, the samples preserved with 33% v/v acetonitrile
produced stable DOSS signals up to 25 h. Therefore,
dispensing 10.0 mL seawater + 5.0 mL acetonitrile into a
20 mL glass vial (33% acetonitrile) at the moment of
sample collection allows for sample storage and transport
to the laboratory with minimal losses.



















