
5
Thermo Scientific Poster Note
•
PN-64078-ASMS-EN-0614S
Conclusions
Automated sample extr
thus decreasing sampl
Insulin MSIA D.A.R.T.’
present at different con
quantification for resea
HRAM MS affords qual
variants present in one
Pinpoint software versi
confirmation, and quan
Reduced complexity aff
LC/MS analysis times.
An LLOD < 15 pM and
were achieved.
Intra- and inter-day rep
MSIA workflow highly r
References
1. Thevis, M, Thomas, A.,
27(1)
, 35-50.
Acknowledgem
The authors would like to than
UK for the donation of the ins
ogs
ntification of insulin and its
n protocols that result in their lack
ulin MSIA workflow described
pg/mL) for the intact variants in
are shown in Figure 4. Tables 1
nd intra-day CVs of < 3%
ecoveries of 96
–
100% (Table 5).
flow significantly reduces the
shorter LC gradients, and,
For Research Use Only. Not
Humulin is a registered trademark of Eli Lil
trademarks are the property of Thermo Fis
This information is not intended to encoura
intellectual property rights of others.
pidra. Lantus and Apidra were
ons. The endogenous insulin
same amount of donor plasma
us insulin remains static. All
response.
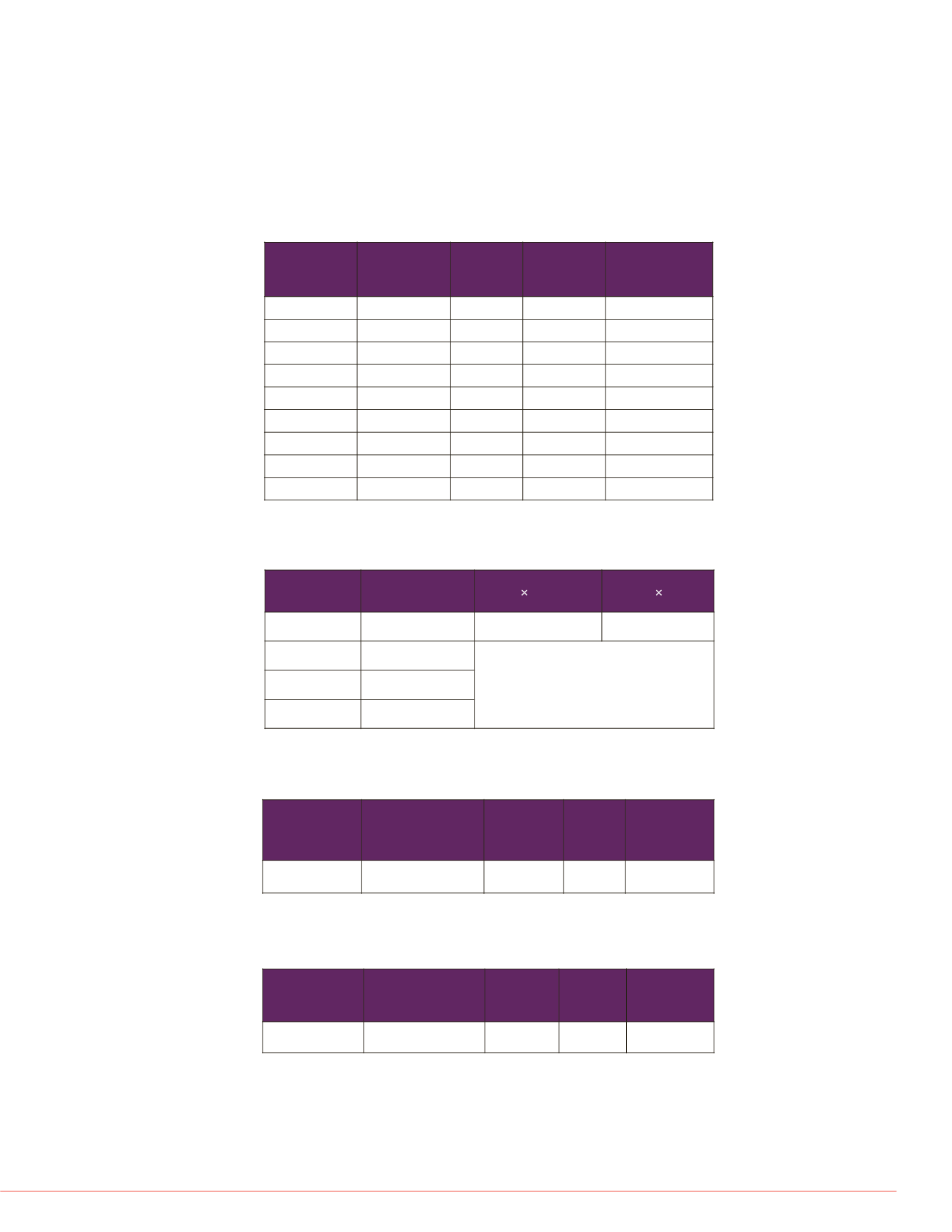
TABLE 1. Limit of quantification
STD Conc.
(pM)
Mean
(5 Curves)
StDev
%CV
Accuracy
0
7.42
1.02
7.5
10.56
0.95
9.04%
40.80%
15
16.78
1.42
8.46%
11.87%
30
28.96
1.12
3.85%
-3.46%
60
58.41
1.61
2.75%
-2.66%
120
115.93
1.96
1.69%
-3.39%
240
232.65
2.80
1.20%
-3.06%
480
473.25
14.41
3.04%
-1.41%
960
963.31
6.47
0.67%
0.34%
TABLE 2. Limit of detection
STD Conc.
(pM)
Mean Total File
Area
4
StDev
Plus 4
StDev
0
2.37E+05
2.20E+05
4.57E+05
7.5
2.80E+05
15
4.79E+05
30
8.93E+05
TABLE 5. Spike and recovery
Sample
Spike Conc.
(pM)
Neat_1
0.00
Neat_2
Neat_3
Low_1
19.50
Low_2
Low_3
Medium_1
199.50
Medium_2
Medium_3
High_1
919.50
High_2
High_3
TABLE 3. Intra-day repeatability
STD Conc.
(pM)
Mean
(3 Controls x
5 Curves)
StDevp
%CV
Accuracy
50.00
51.21
1.33
3
2.43%
TABLE 4. Inter-day repeatability
STD Conc.
(pM)
Mean
(3 Controls x
5 Curves)
StDevp
%CV
Accuracy
50.00
51.07
0.81
2
2.15%
Method Characteristics for the MSIA Insulin Research Workflow
The LLOQ for the insulin MSIA research workflow is 15 pM (highlighted in red in
Table 1), which was determined as the lowest concentration where we could achieve a
%CV of <20% and an accuracy within ±20%.
An LOD of 15 pM (highlighted in red in Table 2) was also achieved for the insulin MSIA
workflow. The LLOD was determined as the lowest concentration where the mean total
area was greater than four standard deviations of the background signal added to the
mean total area for the blank.
insulin variants. Apidra
™
) ,
and porcine as the internal
and detected simultaneously.
state, and shows all three
ree displayed insulin variants.
1600 1700 1800 1900 2000
60 1162 1164 1166 1168 1170 1172 1174
m/z
Apidra
Humulin® S
Apidra
TM
+ Na
+
Apidra
TM
+ K
+
2x + 0.1087
0.996
y = 0.0103x + 0.463
R²= 0.9883
400
500
er Sample (pM)



















